Session Information
Date: Sunday, November 12, 2023
Title: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: ACR gout treatment guidelines recommend a target serum urate (SU) of < 6 mg/dL and anti-inflammatory flare prophylaxis for at least 3-6 months after initiating urate-lowering therapy (ULT). However, optimal targets for SU are not well defined. We investigated gout flare rates based on repeated measurements of SU levels in a randomized controlled trial of ULT, accounting for loss to follow up.
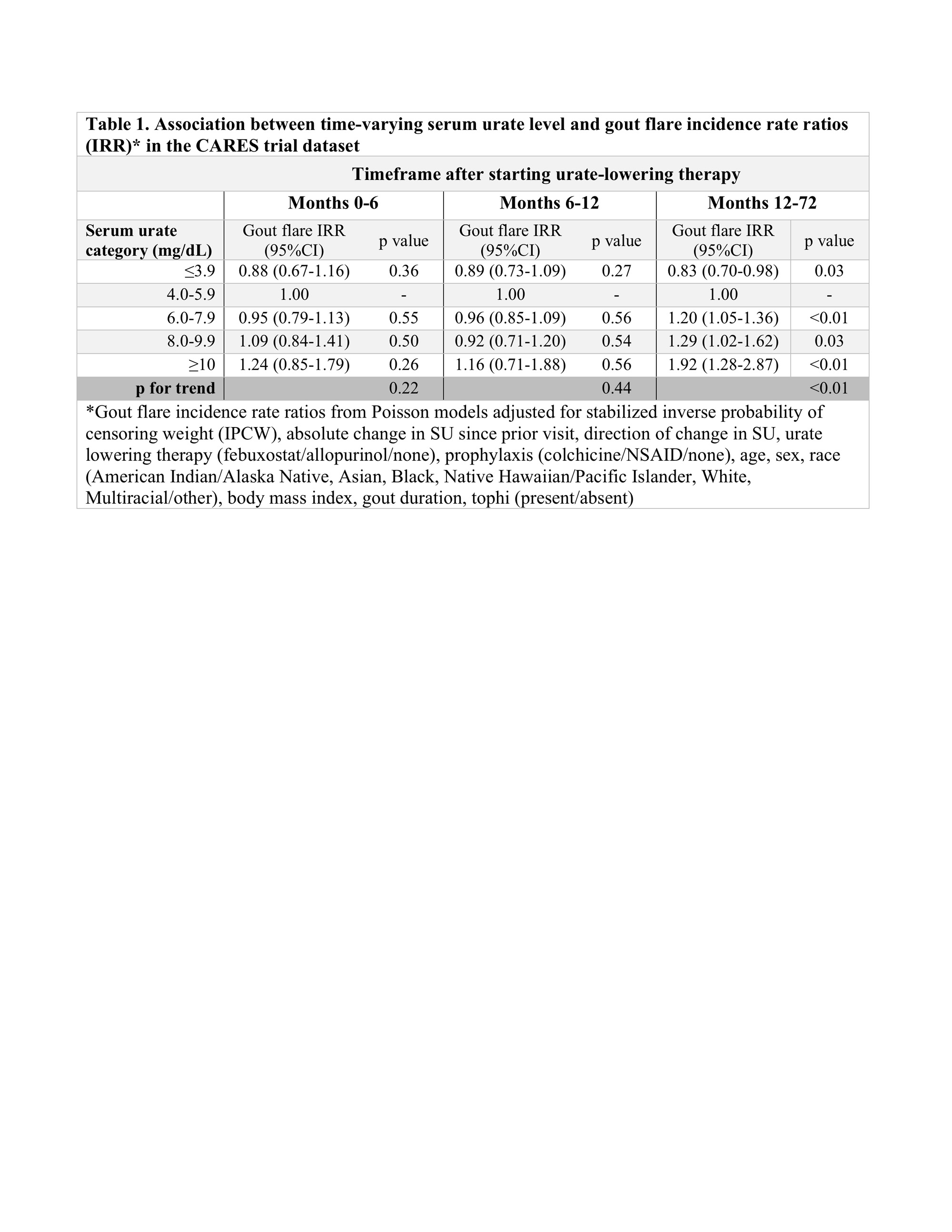
Methods: We performed a secondary analysis using data from the Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout (CARES) trial. CARES participants were randomized to febuxostat or allopurinol, titrated for a target SU < 6 mg/dL; 45% did not complete all study visits. Participants received gout flare prophylaxis for 6 months with colchicine 0.6 mg daily or naproxen 250 mg twice daily if colchicine was not tolerated. For this analysis, participants were followed from month 0 (randomization) to the earliest of death, last completed visit (drop out), or end of study. SU levels were assessed at months 0, 3, 6 and then every 6 months and categorized as ≤3.9, 4.0-5.9, 6.0-7.9, 8.0-9.9, and ≥10 mg/dL. The primary outcome was self-reported gout flare in each 3- or 6-month interval. More than 1 flare/interval was permitted if they were separated by ≥14 days. Baseline variables were used to derive inverse probability of censoring weights (IPCW) to account for censoring (drop-out or death). Poisson regression models included stabilized IPCW weights and estimated gout flare incidence rate ratios (IRR) by time-varying SU category, adjusting for change in SU from prior visit, flare prophylaxis, ULT, age, sex, race, body mass index, gout duration, and tophi. Models were performed for months 0-6, 6-12, and 12-72.
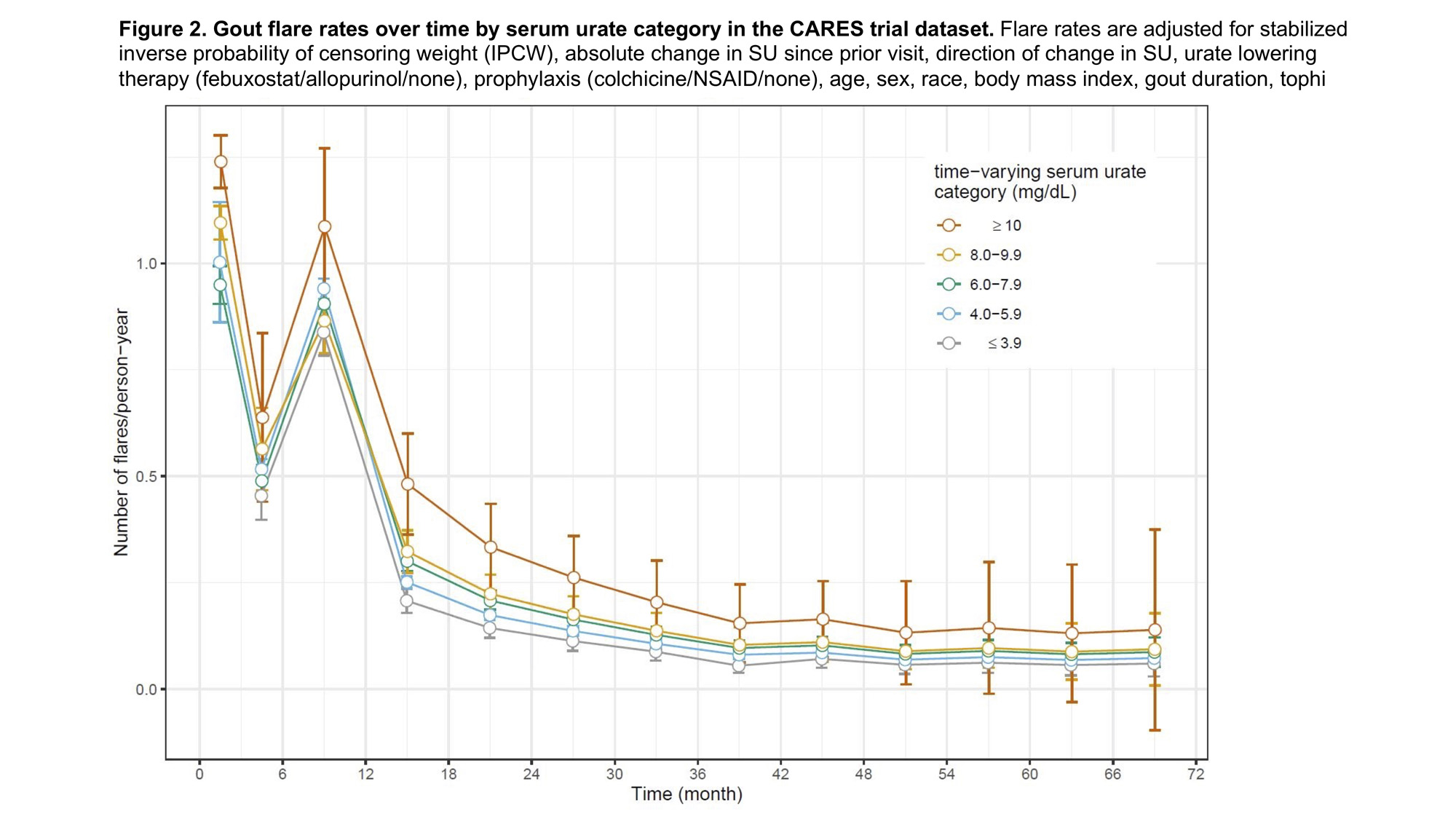
Results: Among 6183 participants in this analysis, median age was 65 (IQR 58-71) years and 84.0% were male. Median follow-up was 32 months. At month 0, median SU was 8.6 (IQR 7.6-9.7) mg/dL. Seventy-one percent achieved SU < 6 mg/dL by month 3, and this percentage increased slightly over time among retained participants (Figures 1A & 1B). Gout flare rates were highest during nearly all intervals when SU ≥10 mg/dL and lowest when SU ≤3.9 mg/dL (Figure 2). Peak gout flare rates for all SU categories were observed between months 0-3, coinciding with the initiation of ULT and greatest change in SU. A second spike in gout flares occurred in all SU groups between months 6-12, coinciding with discontinuation of prophylaxis (Figure 2). In the initial year of ULT, flare rates did not significantly differ between SU groups, but flare rates were consistently highest when SU ≥10 mg/dL. During months 12-72, in adjusted analyses with IPCW weights, a dose-response relationship was observed between SU category and flare rate. Compared with SU 4.0-5.9 mg/dL, significantly lower flare rates were observed when SU ≤3.9 mg/dL and significantly greater rates when SU ≥10 mg/dL (p for trend < 0.01) (Table 1).
Conclusion: Gout flare rates were persistently higher when SU ≥6 mg/dL compared to SU at target after the first year of ULT, after accounting for censoring. These data suggest a potential benefit of achieving very low SU levels (≤3.9 mg/dL) and consideration of a longer duration of prophylaxis to reduce gout flares.
To cite this abstract in AMA style:
Tedeschi S, Hayashi K, Zhang Y, Choi H, Solomon D. Identifying Optimal Serum Urate Levels to Reduce Gout Flares in Patients Taking Urate Lowering Therapy: A Post-hoc Cohort Analysis of CARES with Consideration of Drop-out [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/identifying-optimal-serum-urate-levels-to-reduce-gout-flares-in-patients-taking-urate-lowering-therapy-a-post-hoc-cohort-analysis-of-cares-with-consideration-of-drop-out/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identifying-optimal-serum-urate-levels-to-reduce-gout-flares-in-patients-taking-urate-lowering-therapy-a-post-hoc-cohort-analysis-of-cares-with-consideration-of-drop-out/