Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune Checkpoint Inhibitor (ICI)-induced myositis (ICI-myositis) is a rare, but potentially fatal complication of ICI therapy.Electronic health record (EHR) databases are essential to accrue sufficient patients for clinical investigations regarding phenotypical presentations, associated risks, prognosis, and management and have not yet been fully employed in this area of research.To meet this critical need, this study assessed the factors most effective in identifying ICI-myositis in the Veterans Health Administration (VHA) with the goal of developing an algorithm to accurately identify these patients.
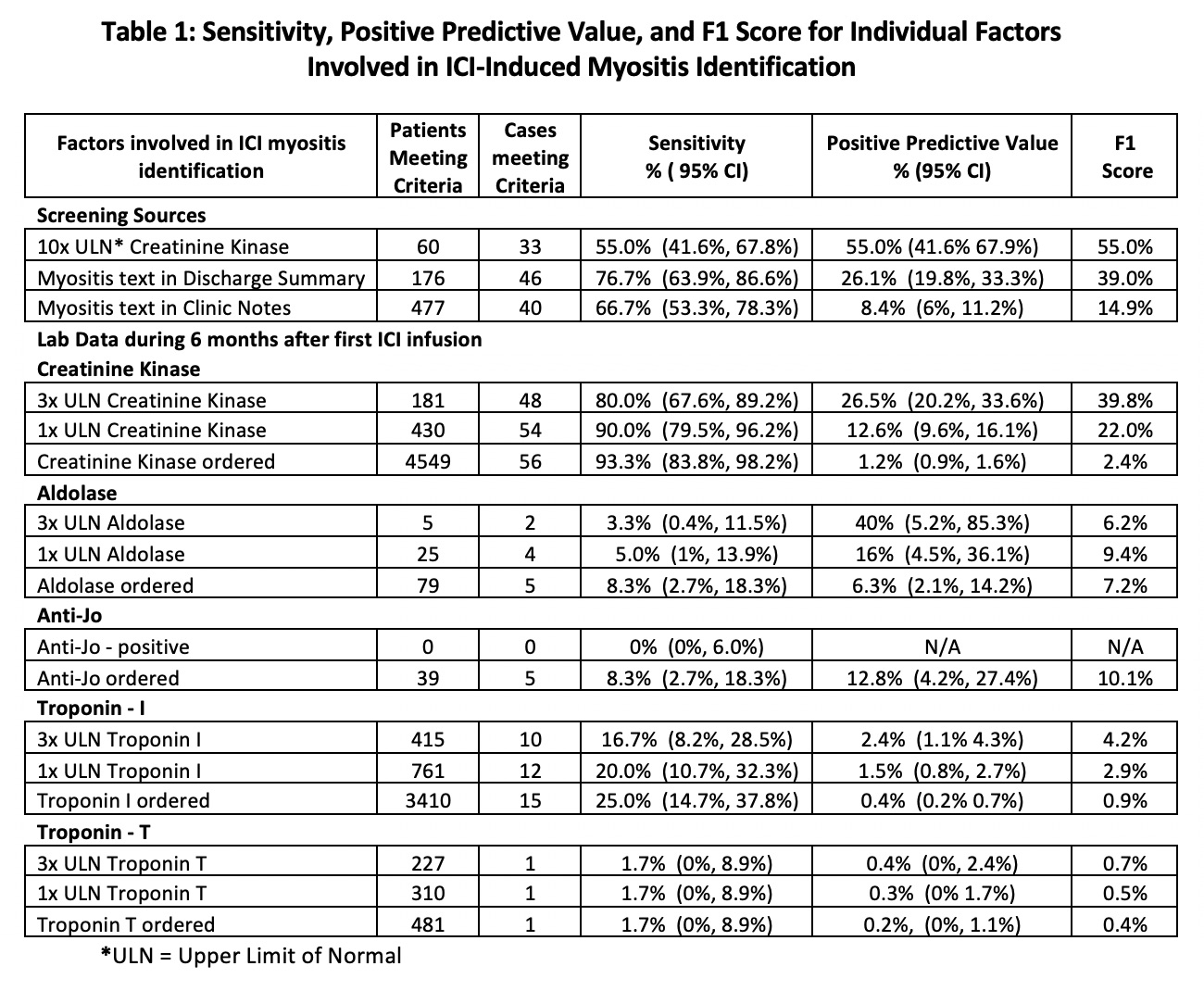
Methods: The VA Corporate Data Warehouse (CDW) containing clinical, laboratory, pharmacy, and administrative records from the VHA’s EHR, identified Veterans treated with an ICI between 6/2011 and 2/2023.Patients at high risk for ICI-myositis underwent electronic medical record review.High risk was defined by having one or more of the following: creatine kinase (CK) over 10 times the upper limit of normal (ULN) (312 U/L) within 6 months after the first ICI, the term “myositis” in a discharge summary text any time after first ICI, and/or the term “myositis” in a progress note text within six months after ICI treatment. Criteria for diagnosis of ICI-myositis were a provider ICI-myositis diagnosis in clinical notes and laboratory evidence of myositis without alternate etiologies.Levels of CK, aldolase, troponin, and Anti-Jo1 antibodies during 6 months after first ICI infusion were extracted from the CDW.Sensitivity, positive predictive value (PPV), and F1 score for individual variables and combinations of variables were calculated in comparison to the gold standard chart review diagnosis.
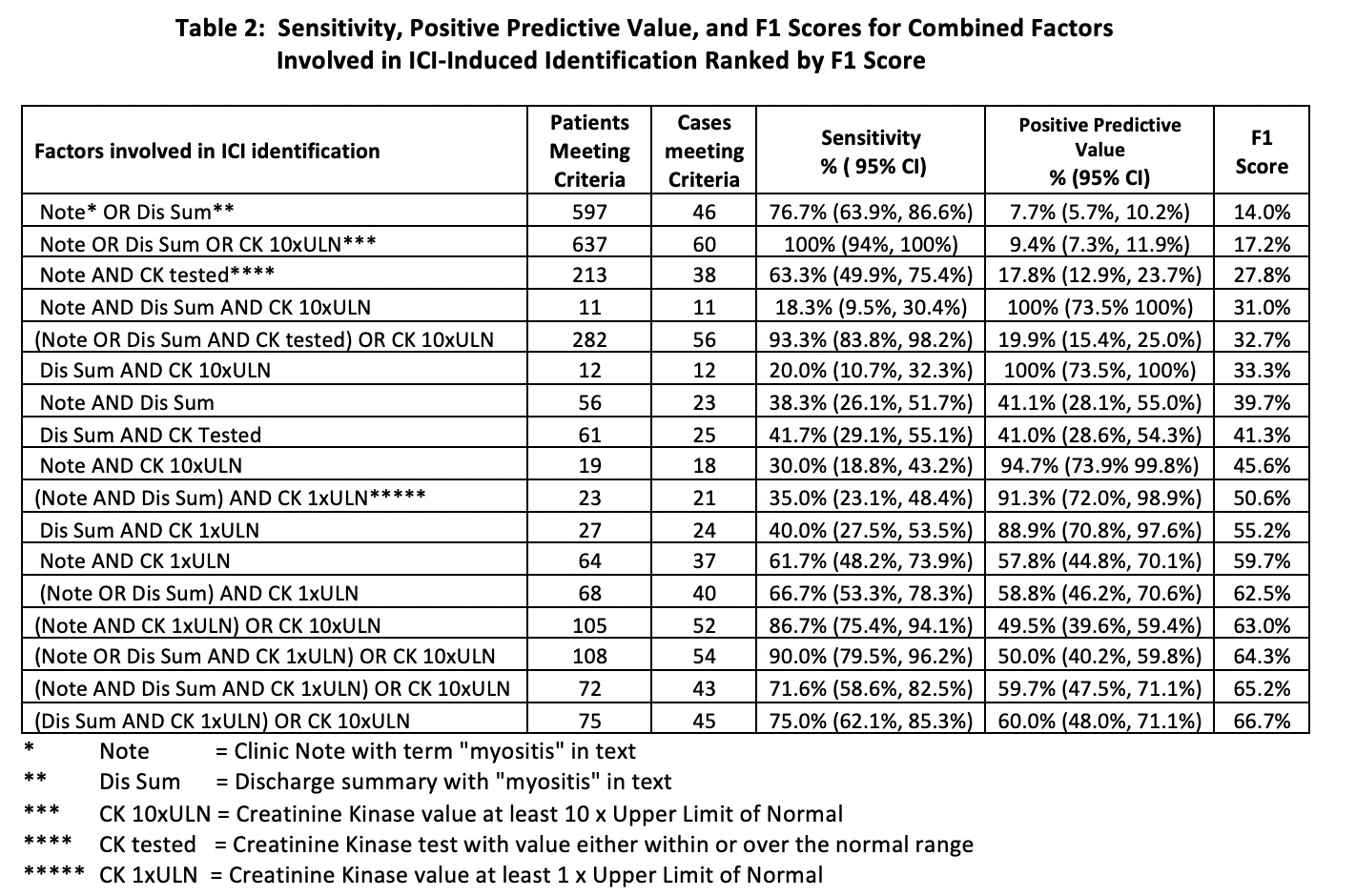
Results: In the 29,562 Veterans receiving an ICI, 60 (0.2%) patients were identified with ICI-myositis.In the 60 patients with CK 10xULN, 33 had ICI-myositis with a sensitivity of 55% and PPV of 55% with F1 score of 55% which was the highest F1 score of any single variable.In the 176 patients with a discharge summary with “myositis” and 477 patients with “myositis” in a clinic note the sensitivities were 76.7% and 66.7% and the PPVs were 26.1% and 8.4% with F1 score of 39.0% and 14.9% respectively.There was a wide range of sensitivity, PPV, and F1 score with the individual lab values (Table 1).The combination of factors demonstrated increased sensitivity, PPV, and F1 score with five combinations having an F1 score greater than 60% which was much higher than the F1-scores seen with individual elements (Table 2).
Conclusion: The multiple factors evaluated for the identification of ICI-myositis had varying degrees of performance with factor combinations performing best. Building on this foundational work, future work will detect and include additional clinical and laboratory factors to enhance the effectiveness of an algorithm to identify ICI-myositis patients in large databases. This work suggests that similar methods may be used to identify other immune related adverse events which can be used for further epidemiological studies.
To cite this abstract in AMA style:
Braaten T, Rubino S, Sauer B, Rojas Jr J, Kunkel G, Walsh J, Patel S, Cannon G. Identification of Immune Checkpoint Inhibitor-Induced Myositis Patients Using Electronic Health Records Is Most Successful When Employing Multiple Data Elements [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/identification-of-immune-checkpoint-inhibitor-induced-myositis-patients-using-electronic-health-records-is-most-successful-when-employing-multiple-data-elements/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-immune-checkpoint-inhibitor-induced-myositis-patients-using-electronic-health-records-is-most-successful-when-employing-multiple-data-elements/