Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: During the COVID-19 pandemic, rapid guidelines by the National Institute for Health and Care Excellence (NICE) in the United Kingdom[1] recommended consideration of switching from intravenous (IV) treatment to subcutaneous (SQ) form to minimise the risk of exposure. The aim of this study is to assess patient-reported outcomes of those who have been switched to SQ form from IV abatacept (ABT) and tocilizumab (TCZ).
Methods: We analysed RA patients at a large rheumatology centre fulfilling the 2010 ACR/EULAR classification criteria who were switched from IV to SQ ABT and TCZ. Patients who have not responded to the SQ form in the past were not switched. We compared Multi-Dimensional Health Assessment Questionnaire (MD-HAQ) scores immediately before and 3 months after the IV to SQ switch. RAPID3 was calculated using three components of MD-HAQ (functional status, pain, and global health). Patients were also asked whether they would prefer their current SQ regimen to continue or whether they would like to revert to IV. Reasons for patient preference to revert to IV were also captured.
Results: 32 patients were switched from IV to SQ [14 (43.7%) ABT and 18 (56.3%) TCZ]. Of the 32 patients who switched, 29 responded to our questionnaires. The majority of patients were in DAS28-ESR clinical remission or low disease activity prior to the switch (mean DAS28-ESR 3.00 for ABT, 2.15 for TCZ).
Unexpectedly, 77% (10/13) in the ABT group and 88% (14/16) in the TCZ group, expressed a preference to return to their IV regimen (Fig. 1). In the ABT group, patient preference to revert to IV was primarily due to worsening symptoms of joint pain/stiffness since the switch to SQ (symptom recurrence described in the last 2 days of weekly injection) (Table 1). This, in turn, is associated with a negative impact on function, represented by the statistically significant increase in MD-HAQ functional status score from 3.662 to 4.408 (p= 0.042), with higher values reflecting negative patient experiences (Fig. 2). We did not find any significant differences in the other 2 components of MD-HAQ and RAPID3.
In the TCZ group however, although the majority expressed a preference to revert to IV, no statistically significant differences were noted in MD-HAQ and RAPID3. 50% (7/14) preferred to revert to IV due to worsening of symptoms, whilst 35.7% (5/14) requested to revert to IV as they preferred the face-to-face interaction with a healthcare professional.
Conclusion: This was a small study but nevertheless indicates that the vast majority of RA patients who switched from IV to SQ ABT and TCZ expressed a preference to switch back. For ABT, this decision was associated with worsening of their functional status whereas for TCZ, although we did not find any statistical significant difference in MD-HAQ and RAPID3, the most commonly reported reasons for request to revert to IV were either worsening of symptoms or the benefit of interaction with a healthcare professional with the IV route of administration. These results suggest that IV and SQ formulations should not be considered as equivalent medications for RA.
- COVID-19 rapid guideline: rheumatological autoimmune, inflammatory and metabolic bone disorders. Published: 3 April 2020 www.nice.org.uk/guidance/ng167.
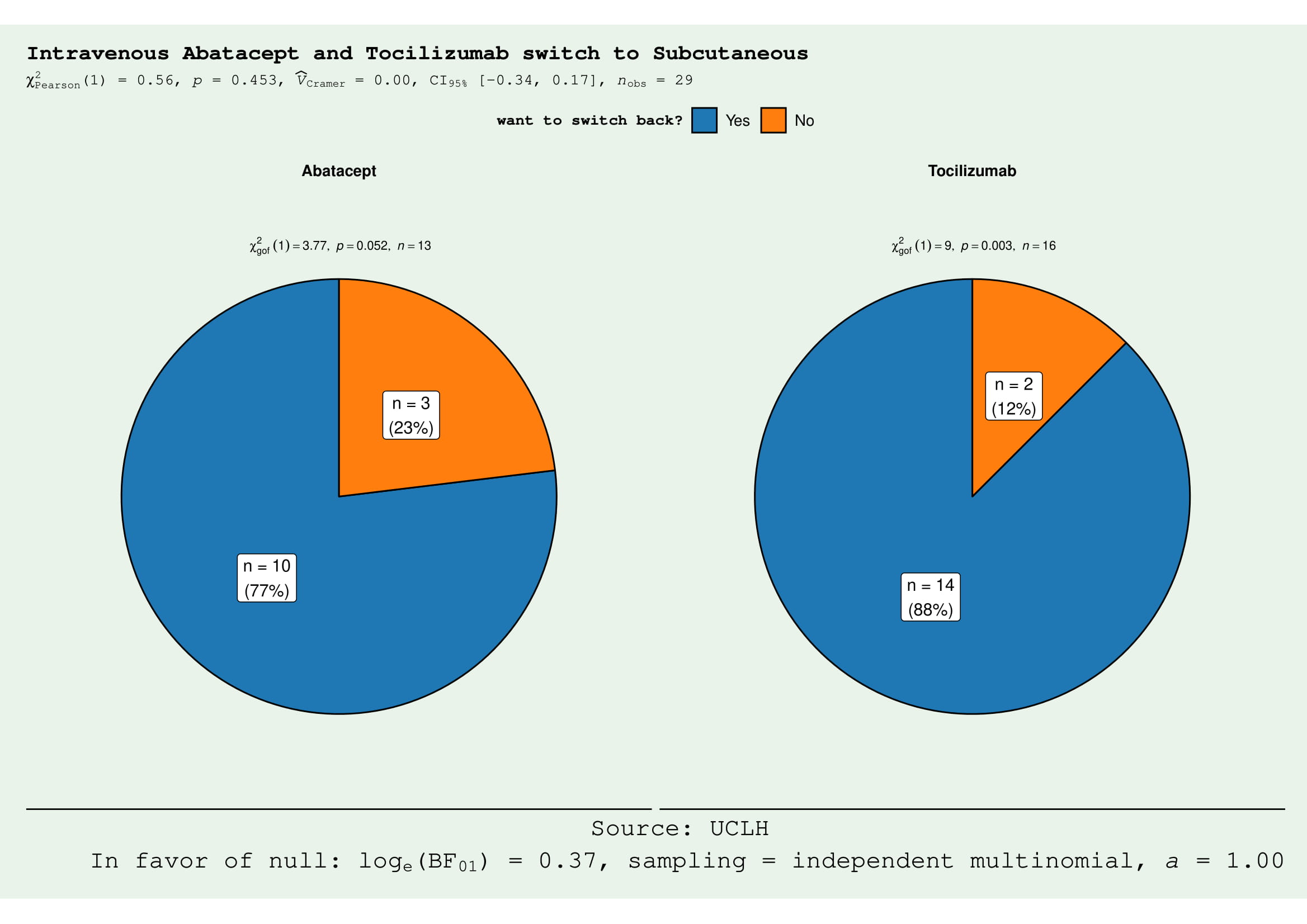 Figure 1. The majority of the patients in the abatacept (ABT) and tocilizumab (TCZ) groups preferred to switch back from subcutaneous (SQ) to intravenous (IV) form. In ABT group, 10/13 wanted to switch back (χ2 3.77, p = 0.052). In the TCZ group, 14/16 would like to switch (χ2 9, p = 0.003).
Figure 1. The majority of the patients in the abatacept (ABT) and tocilizumab (TCZ) groups preferred to switch back from subcutaneous (SQ) to intravenous (IV) form. In ABT group, 10/13 wanted to switch back (χ2 3.77, p = 0.052). In the TCZ group, 14/16 would like to switch (χ2 9, p = 0.003).
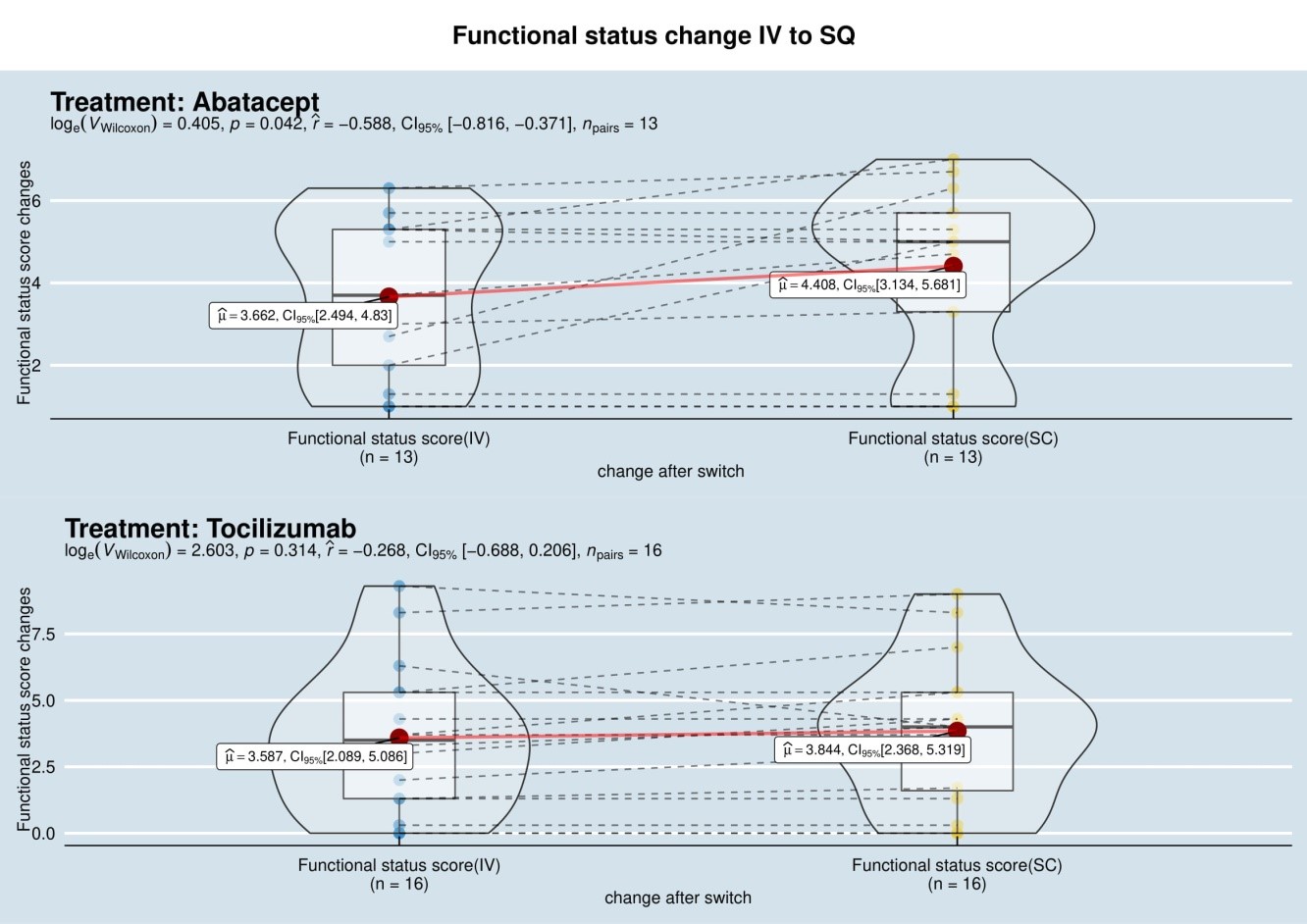 Figure 2. Switching Abatacept (ABT) to subcutaneous (SQ) form, resulted increase in mean score to 4.408 from 3.66 (p = 0.042).
Figure 2. Switching Abatacept (ABT) to subcutaneous (SQ) form, resulted increase in mean score to 4.408 from 3.66 (p = 0.042).
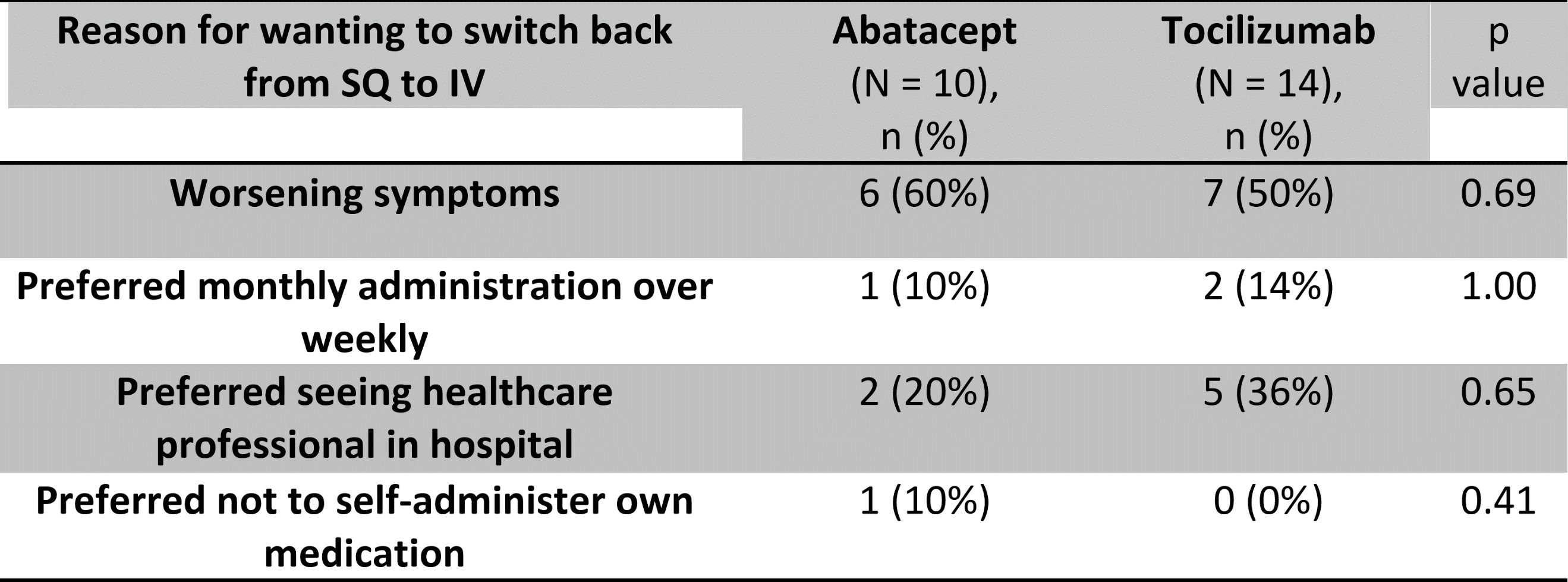 Table 1. Reasons for wanting to switch back from SQ to IV.
Table 1. Reasons for wanting to switch back from SQ to IV.
To cite this abstract in AMA style:
Gupta R, Shipa M, Yeoh S, Buck P, Ehrenstein M. “I Want to Switch Back”: Real-world Experience of Switching Intravenous Abatacept and Tocilizumab to Subcutaneous Injection During the COVID-19 Pandemic [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/i-want-to-switch-back-real-world-experience-of-switching-intravenous-abatacept-and-tocilizumab-to-subcutaneous-injection-during-the-covid-19-pandemic/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/i-want-to-switch-back-real-world-experience-of-switching-intravenous-abatacept-and-tocilizumab-to-subcutaneous-injection-during-the-covid-19-pandemic/
