Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Hydroxychloroquine (HCQ) is an extensively used drug in immune-mediated diseases (IMID). Despite its general safety, HCQ can cause serious toxicity such as heart conduction disorders. Atrioventricular block (AVB) is an underrecognized adverse effect that can potentially cause significant morbidity and mortality.
The aim of this study was to analyze incidence, presentation, and characteristics of HCQ-induced AVB in IMID.
Methods: Open-label single center study of 293 patients with IMID treated with HCQ for at least 3 months. Electrocardiograms were analyzed at baseline and during HCQ treatment. In addition, a comparative study between patients with and without AVB was conducted.
Results: We studied 293 patients (270 women/23 men; mean age 59.7±14.7 years). Underlying IMID were systemic lupus erythematosus (n=109, 40.6%); undifferentiated connective-tissue disease (n=70, 23.9%), Sjögren’s syndrome (n=70, 23.9%), antiphospholipid syndrome (n=31,10.6%) and other IMID (n=13, 4.4%). HCQ was used for 4.1±3.5 years.
After 11.8±8.9 years of follow-up (HCQ mean cumulative dose: 979.7±272.1 g). AVB was observed in 19 out of 293 (6.5%) patients: 16 (84.2%) were first-degree AVB and 3 (15.8%) complete AVB. 4 patients with AVB were treated with a permanent pacemaker.
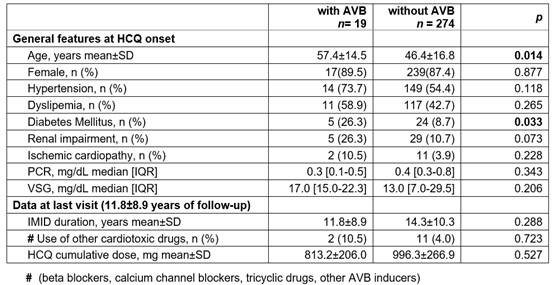
A comparative study between patients with and without AVB was performed (TABLE). Patients with AVB were older (p=0.014) and had a higher incidence of diabetes mellitus (p=0.033). HCQ cumulative dose and duration of IMID were similar in both groups (p >0.05).
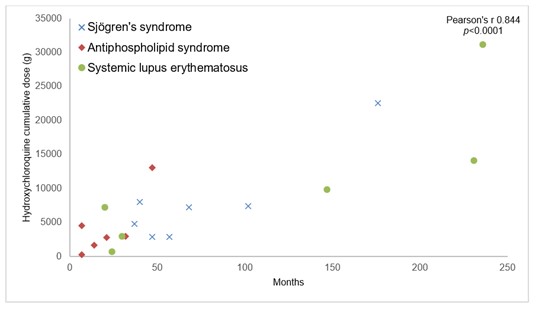
Presentation of atrioventricular block according to hydroxychloroquine cumulative dose and treatment duration is shown in FIGURE. Most of AVB happened in the first 40 months, regardless of HCQ cumulative dose.
Other adverse effects of HCQ were retinopathy (n=16, 5.4%), gastrointestinal alterations (n=14, 4.8%), cutaneous alterations (n=14, 4.8%), allergic reactions (n=4, 1.4%) and myopathy (n=1, 0.3%).
Conclusion: AVB was observed in 6.5% of patients with IMID treated with HCQ. Most AVB were first-degree AVB. HCQ increased the risk of developing an AVB in patients with IMID regardless of HCQ cumulative dose or underlying disease duration. Older patients with diabetes mellitus had a higher risk of developing an AVB.
To cite this abstract in AMA style:
Herrero-Morant A, Margarida-de Castro A, Pérez-Barquín R, Zubiaur-Zamacola J, gonzalez-Gay M, Blanco R. Hydroxycloroquine-Induced Atrioventricular Block in Inmune-Mediated Diseases. Single University Center Study of 293 Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/hydroxycloroquine-induced-atrioventricular-block-in-inmune-mediated-diseases-single-university-center-study-of-293-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxycloroquine-induced-atrioventricular-block-in-inmune-mediated-diseases-single-university-center-study-of-293-patients/