Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The Study of Antimalarials in Incomplete Lupus Erythematosus (SMILE, NCT03030118) was a double-blind, randomized, placebo-controlled study of hydroxychloroquine (HCQ) to prevent the development of lupus in people at risk. Participants had an ANA of ≥1:80 and one or two but not three 2012 SLICC criteria for the classification of SLE. They were randomized to either 400 mg/d of HCQ (200 mg for participants under 40kg) or matching placebo (PBO). They were followed for up to 24 months for the development of new SLICC criteria. 187 individuals were enrolled in the trial. Biospecimens were collected every three months for mechanistic studies. The objective of this study was to assess the effect of HCQ on the level of antibodies to a panel of 120 potential autoantigens.
Methods: Serum was collected from SMILE participants at baseline and every three months for a total of 24 months or until they met SLICC classification criteria for SLE. IgG and IgM antibodies to 120 antigens previously demonstrated in patients with SLE and other autoimmune diseases were quantified by fluorescence intensity on antigen microarrays. Signal intensity was normalized to total IgG or IgM for each sample and log2transformed for analysis. Levels of individual autoantibodies were compared at baseline and at the last study visit and significance determined using a paired t-test. Nominal p values were corrected for multiple comparisons by Benjamini-Hochberg at a False-Discovery Rate of 0.05.
Results: 152 of the 187 SMILE participants had informative pairs of serum samples. These included 46 individuals who got HCQ and 50 who got PBO and did not develop new SLICC criteria over 24 months (“Non-Progressors”, NP); 16 individuals who got HCQ and 16 who got PBO who developed a second SLICC criterion in addition to the ANA (“Progressors”, PR); 12 individuals who got HCQ and 12 who got PBO who developed criteria for classification of lupus (“Developed SLE”, DS).The Progressors and Developed SLE individuals were combined (PRDS) for comparison to the NP. In the group of participants who received HCQ, 93 of 120 antibodies levels were significantly lower at the end of the study compared to 6 of 120 antibodies in the group that received PBO (p = 2.2×10-6, Fisher’s exact test). Table 1 shows the results by treatment group and study outcome. No IgG antibody levels were significantly increased at the end of the study and no IgM antibody levels were significantly affected by hydroxychloroquine in the SMILE study. The median decrease in autoantibody level was 13.4% for the NP participants and 19.4% for the PRDS participants. The maximum decreases were 46.4% for the NP participants and 52.7% for the PRDS participants. Decreases in mean autoantibody levels in response to hydroxychloroquine varied. The mean decreases in antibody titers for select antigens are shown in Table 2.
Conclusion: In a cohort of individuals at risk for the development of systemic lupus erythematosus, treatment with hydroxychloroquine resulted in significant decreases in autoantibody levels measured against a diverse panel of antigens. This feature of treatment with hydroxychloroquine may be a factor in the clinical efficacy of this agent.
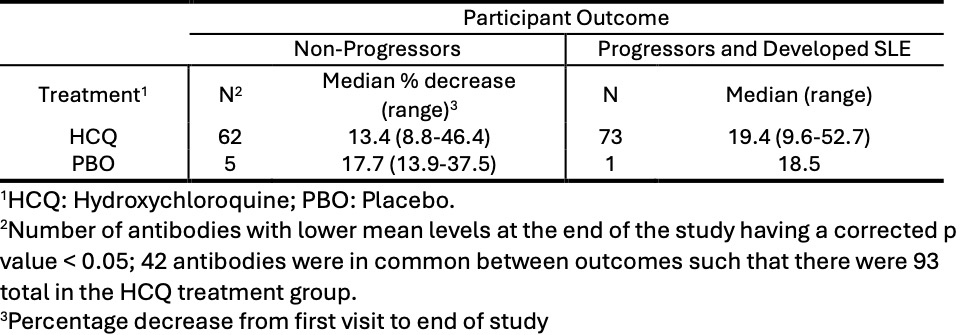 Table 1: Effect of Hydroxychloroquine on Autoantibody Levels
Table 1: Effect of Hydroxychloroquine on Autoantibody Levels
.jpg) Table 2: Mean Decreases in Autoantibody levels for Select Antigens in Individuals Receiving Hydroxychloroquine
Table 2: Mean Decreases in Autoantibody levels for Select Antigens in Individuals Receiving Hydroxychloroquine
To cite this abstract in AMA style:
Karp D, Raj P, Zhu C, Liu D, Liao D, Olsen N. Hydroxychloroquine Reduces Autoantibody Levels in Persons at Risk for Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-reduces-autoantibody-levels-in-persons-at-risk-for-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-reduces-autoantibody-levels-in-persons-at-risk-for-systemic-lupus-erythematosus/
