Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is a medication used in SLE during pregnancy. An adverse effect of HCQ includes cardiotoxicity. Hormonal changes during pregnancy may trigger SLE cardiomyopathy. The objective of this study was to examine the association of HCQ use during pregnancy with new onset adverse cardiovascular (CV) outcomes during pregnancy and postpartum in subjects with SLE.
Methods: In this study, we used the Synthetic Derivative, a de-identified copy of the EHR containing billing codes and clinical data spanning 1989-2024. We identified pregnant subjects with SLE and CV outcomes using ICD-9/ICD-10 codes for 1) SLE, 2) labor/delivery, and 3) heart failure (HF)/peripartum cardiomyopathy or CPT codes for cardiac MRI. Charts were manually reviewed by a physician to ascertain SLE clinical diagnosis and pregnancy (inclusion criteria). Subjects without SLE, pregnancy prior to SLE, onset of HF prior to pregnancy, and pregnancies terminating < 20 weeks were excluded. Demographic and clinical characteristics, HCQ use during pregnancy, immunosuppressive therapy (IST), and maternal complications were abstracted. Medical records were reviewed from conception until six months after delivery to identify new onset adverse CV outcomes (HF, arrhythmias, valvular disease, pericarditis, Cor pulmonale) during pregnancy and postpartum. Comparisons were made using Mann-Whitney U test for continuous variables and Fisher’s exact test or X2-test for categorical variables, as appropriate.
Results: Of 131 identified cases, we included 33 pregnant subjects with SLE. Median (IQR) age at delivery was 27 (24–31) years. Seventeen (51%) of them were taking HCQ during pregnancy. Those on HCQ during pregnancy were more likely to have received IST (64% vs 25%, p=0.04) and aspirin (82% vs 37%, p=0.01) during pregnancy, and had lower median glucocorticoid (GC) dose at delivery (10 vs 20 mg/day, p=0.04). Demographic and clinical characteristics are shown in Table 1. Ten (30%) subjects developed new onset CV outcomes during pregnancy and/or the postpartum period, whereas 23 (70%) didn’t have cardiac complications during the follow-up period. Half (n=5) of the subjects with adverse CV outcomes received HCQ during pregnancy (Table 2). All five adverse CV outcomes in the HCQ group were HF, one of whom had peripartum cardiomyopathy with recovery of myocardial function in the postpartum period without HCQ discontinuation. Twenty-two (66.7%) patients developed maternal complications, of whom 50% (n=11) were on HCQ during pregnancy. The use of HCQ was similar between individuals with or without adverse CV outcomes and maternal complications (Table 3). Those with adverse CV outcomes had shorter SLE duration (2 vs 5 years, p=0.03). Individuals with maternal complications were younger at delivery (26 vs 32 years, p=0.008), had higher rates of preterm birth (77% vs 27%, p=0.009), positivity of dsDNA antibody (95% vs 40%, p=0.002), and IST use (63% vs 9%, p=0.001).
Conclusion: In this study, there was no difference in adverse CV and maternal outcomes with or without the use of HCQ among pregnant individuals with SLE. Those with adverse outcomes had shorter SLE duration, were younger, and had more exposure to IST, suggesting higher SLE disease activity.
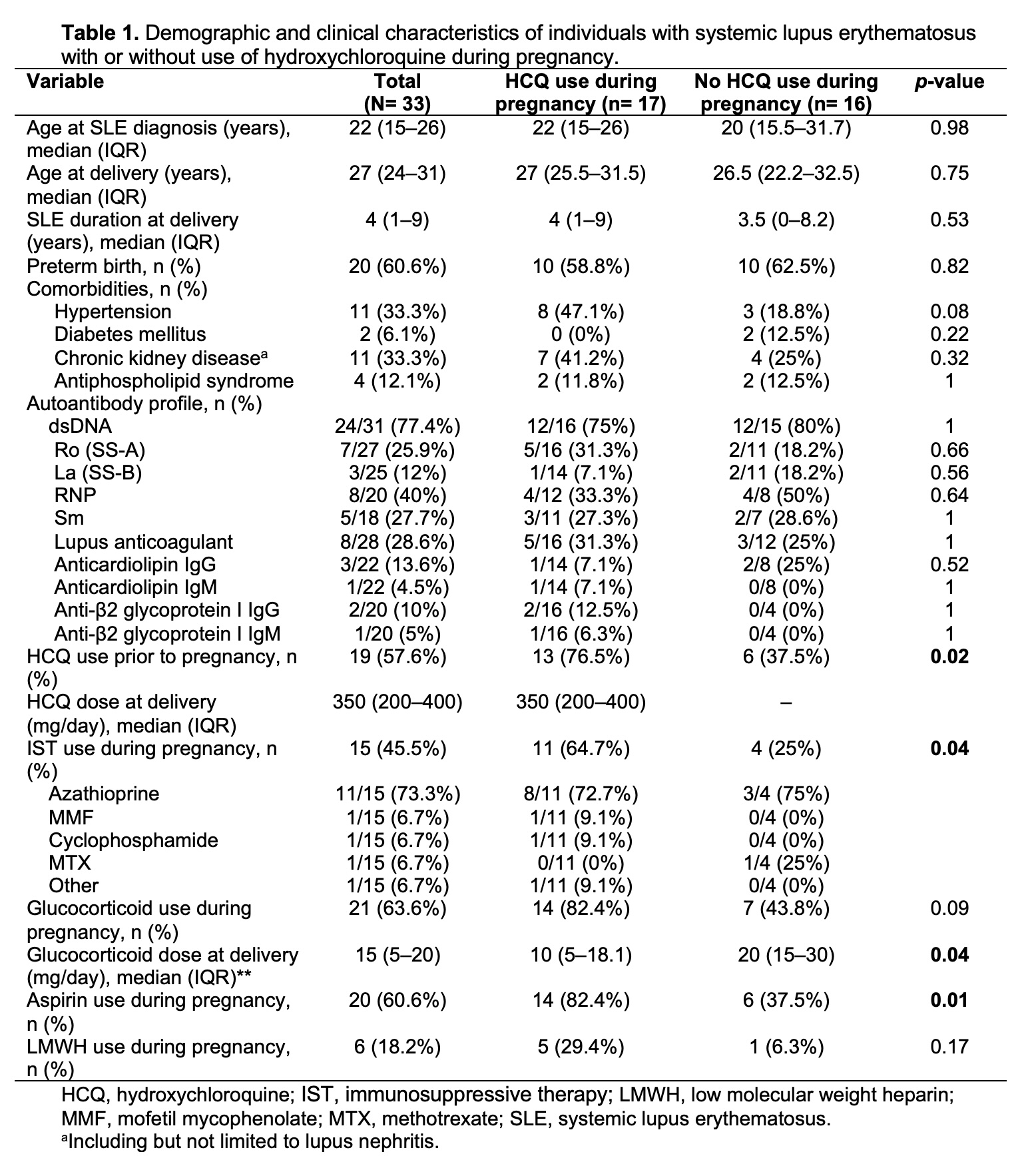 Table 1. Demographic and clinical characteristics of individuals with systemic lupus erythematosus with or without use of hydroxychloroquine during pregnancy.
Table 1. Demographic and clinical characteristics of individuals with systemic lupus erythematosus with or without use of hydroxychloroquine during pregnancy.
.jpg) Table 2. Cardiovascular and pregnancy outcomes of subjects with systemic lupus erythematosus with or without hydroxychloroquine use during pregnancy
Table 2. Cardiovascular and pregnancy outcomes of subjects with systemic lupus erythematosus with or without hydroxychloroquine use during pregnancy
.jpg) Table 3. Demographics and clinical characteristics of pregnant individuals with systemic lupus erythematosus with or without adverse CV outcomes or maternal complications.
Table 3. Demographics and clinical characteristics of pregnant individuals with systemic lupus erythematosus with or without adverse CV outcomes or maternal complications.
To cite this abstract in AMA style:
Gonzalez-Treviño M, Ciosek A, Maldonado G, Deffendall C, Ruiz T, Frech T, Dhital R. Hydroxychloroquine and Adverse Cardiovascular Outcomes in Pregnant Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-and-adverse-cardiovascular-outcomes-in-pregnant-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-and-adverse-cardiovascular-outcomes-in-pregnant-patients-with-systemic-lupus-erythematosus/
