Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Basic Science (0807–0812)
Session Type: Abstract Session
Session Time: 1:30PM-1:45PM
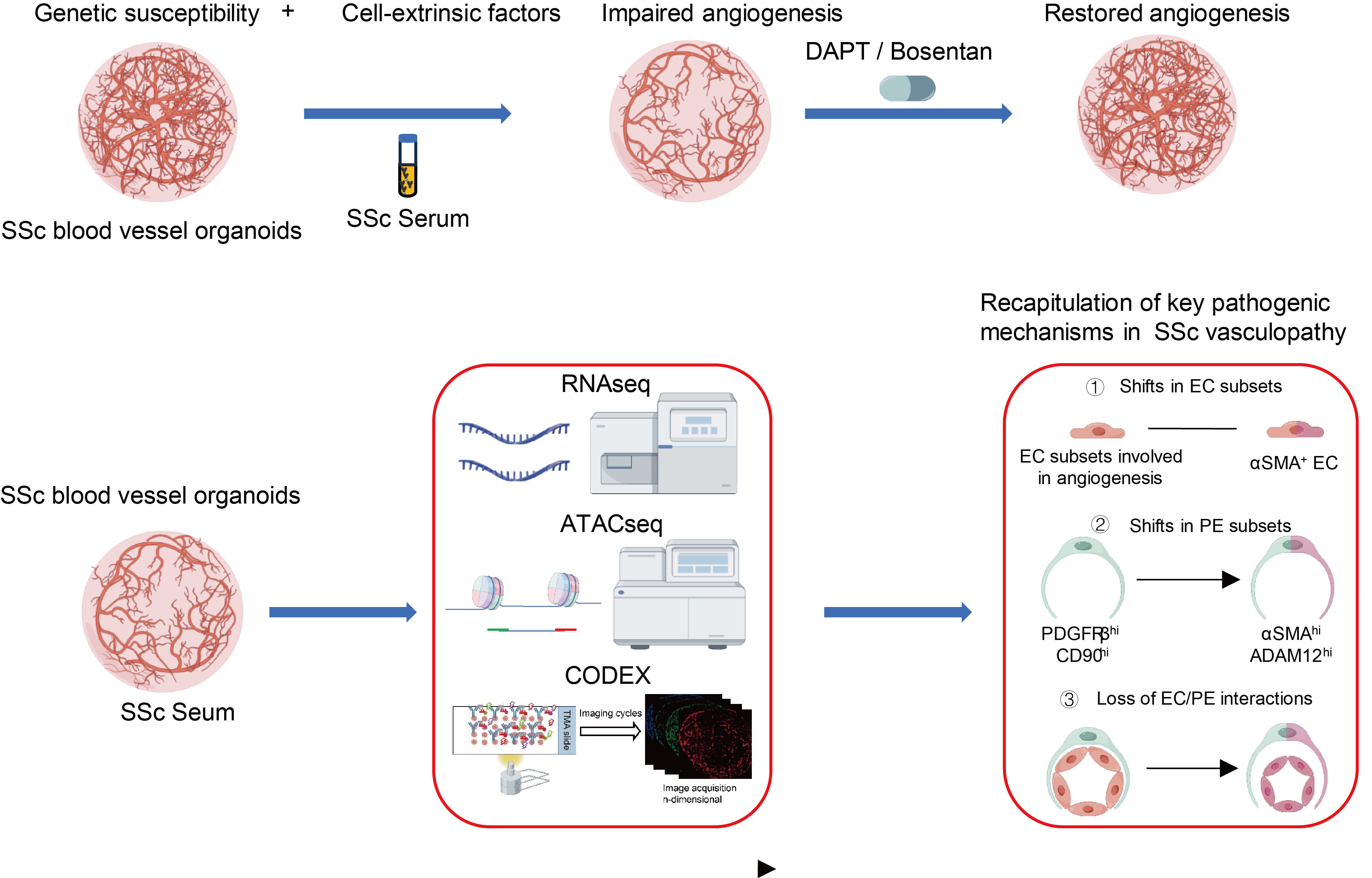
Background/Purpose: While several pathogenic processes involved in vasculopathy in systemic sclerosis (SSc) have been described1-3, the mechanisms that underlie the SSc microvasculopathy remain incompletely understood. Human models of sufficient complexity to recapitulate the key disease-relevant cell types and their interactions are currently lacking. Thus, the preclinical testing of novel therapeutic approaches for SSc microvasculopathy remains challenging.Our study aimed to evaluate whether blood vessel organoids (BVOs) could be employed as a novel, multicellular tissue-like three-dimensional model for studying SSc vasculopathy in vitro.
Methods: BVOs were differentiated from iPSCs of SSc patients (SSc-BVOs) and healthy individuals (healthy-BVOs) and treated with serum from healthy donors or SSc patients with active digital ulcers (SSc_aDU). Angiogenesis parameters, including vessel density, vessel length, and number of junctions, were quantified using AngioTool. Transcriptomic changes and chromatin accessibility were assessed by RNA-seq and ATAC-seq, respectively. Co-detection by indexing (CODEX), a spatial proteomics technique, was employed for cell phenotyping, evaluation of functional markers and of cell-cell interactions. The endothelin receptor antagonist bosentan (BST) and the γ-secretase inhibitor DAPT were tested for their effects in preventing angiogenesis defects in BVOs.
Results: SSc-BVOs, but not healthy-BVOs demonstrated angiogenic defects, with decreased vessel density, vessel length and number of junctions, when exposed to SSc_aDU serum.Exposure of SSc-BVOs to SSc_aDU serum triggers concerted changes at epigenetic, mRNA and protein levels to induce upregulation of endothelial-to-mesenchymal transition (EndMT)-related pathways and proteins, such as αSMA, SNAIL1, TWIST1 and collagen synthesis, and downregulation of markers and pathways related to an endothelial cells (ECs) phenotype, such as CD31, VWF or tight junctions. CODEX analysis demonstrated that BVOs recapitulate ECs and pericyte populations in SSc, along with their disease-specific changes in frequencies. Two αSMA+ ECs subsets were upregulated in SSc BVOs exposed to SSc_aDU serum, as previously shown in SSc4. The Tie2+ ECs lost interactions with pericytes, whereas the two αSMA+ ECs were spatially avoiding pericytes in SSc-BVOs exposed to SSc_aDU serum, recapitulating the impaired ECs-pericytes interactions previously described in SSc5. Treatment with BST and DAPT prevented the angiogenesis defects induced by SSc_aDU serum in SSc-BVOs.
Conclusion: This study demonstrates that SSc-BVOs recapitulate key features of SSc vasculopathy, including impaired angiogenesis and enhanced EndMT, increases in pathologic ECs populations, as well as loss of ECs -pericytes interactions when exposed to SSc_aDU serum. This study further validates SSc-BVOs as a human model system for drug testing and employs this system to demostrate that γ-secretase inhibition is a potential therapeutic approach in SSc microvasculopathy.
 SSc blood vessel organoids reveal mechanisms of vasculopathy and responses to targeted therapy
SSc blood vessel organoids reveal mechanisms of vasculopathy and responses to targeted therapy
To cite this abstract in AMA style:
Xiao Y, Hong X, Zhi L, Li Y, Regensburger M, Marxreiter F, Görg B, Koziel S, Györfi A, Filla T, Bruch P, Tripal P, Adjaye J, Dietrich S, Winkler J, Distler J, Matei A. Human blood vessel organoids as a model of vasculopathy in systemic sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/human-blood-vessel-organoids-as-a-model-of-vasculopathy-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/human-blood-vessel-organoids-as-a-model-of-vasculopathy-in-systemic-sclerosis/
