Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Power Doppler ultrasound (PDUS) allows the visualization of morphological and inflammatory changes of the synovium. The ULTIMATE study (NCT02662985) was the first large, randomized, double-blind, placebo-controlled multi-center PDUS Phase 3b study in psoriatic arthritis (PsA), which utilized the Global OMERACT-EULAR Synovitis Score (GLOESS), a global ultrasound score, to demonstrate the responsiveness of PDUS to detect early, and continuous decrease in the synovitis score.1 However, the ultrasound assessment for GLOESS is time-consuming due to the number of joints assessed. The aim of this analysis was to investigate how well various reduced joint sets predict response to secukinumab, compared with the total number of joints included in the GLOESS.
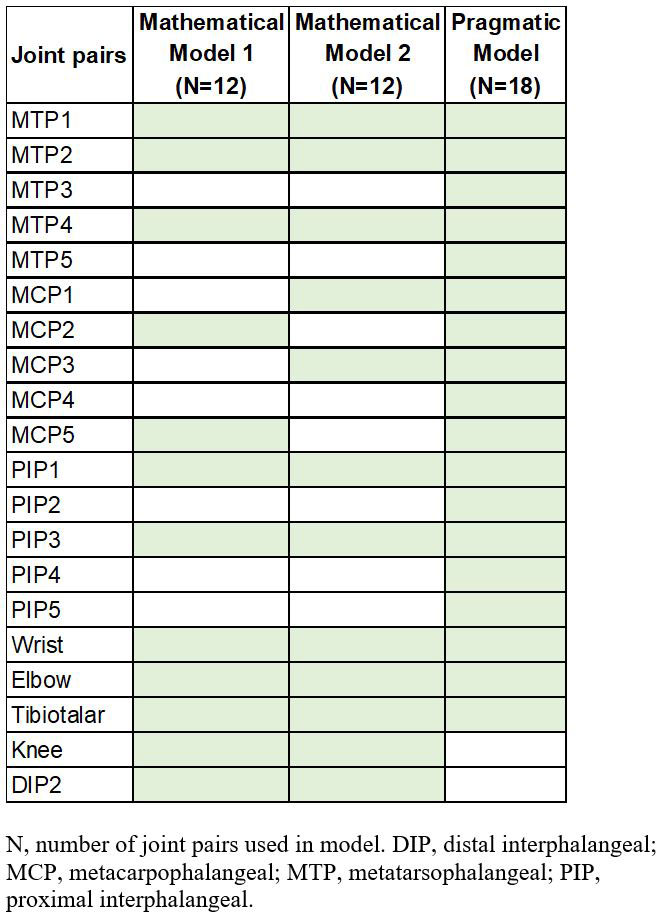
Methods: ULTIMATE was a 52-week study, with a 12-week double-blind, placebo-controlled period followed by 12-week open-label treatment and 6-month extension period.1 In the ULTIMATE trial, GLOESS for the 24 paired joints was calculated, with a potential score ranging between 0 to 144. A Cluster Image Map was constructed to identify highly correlated joint clusters, based on the change from baseline of the composite PDUS scores at joint level. A reduced set of joints was selected based upon either a mathematical approach that used the correlation clusters along with the joint level variations and the joint location (models 1 and 2), or a pragmatic joint set based on the most frequently involved joints in PsA in clinical practice (model 3). This yielded three corresponding models for comparison. Linear models were developed with these reduced joint sets as predictors, based on the data from 60% of patients randomly selected from the ULTIMATE study. The remaining 40% were used for model validation and diagnostics.
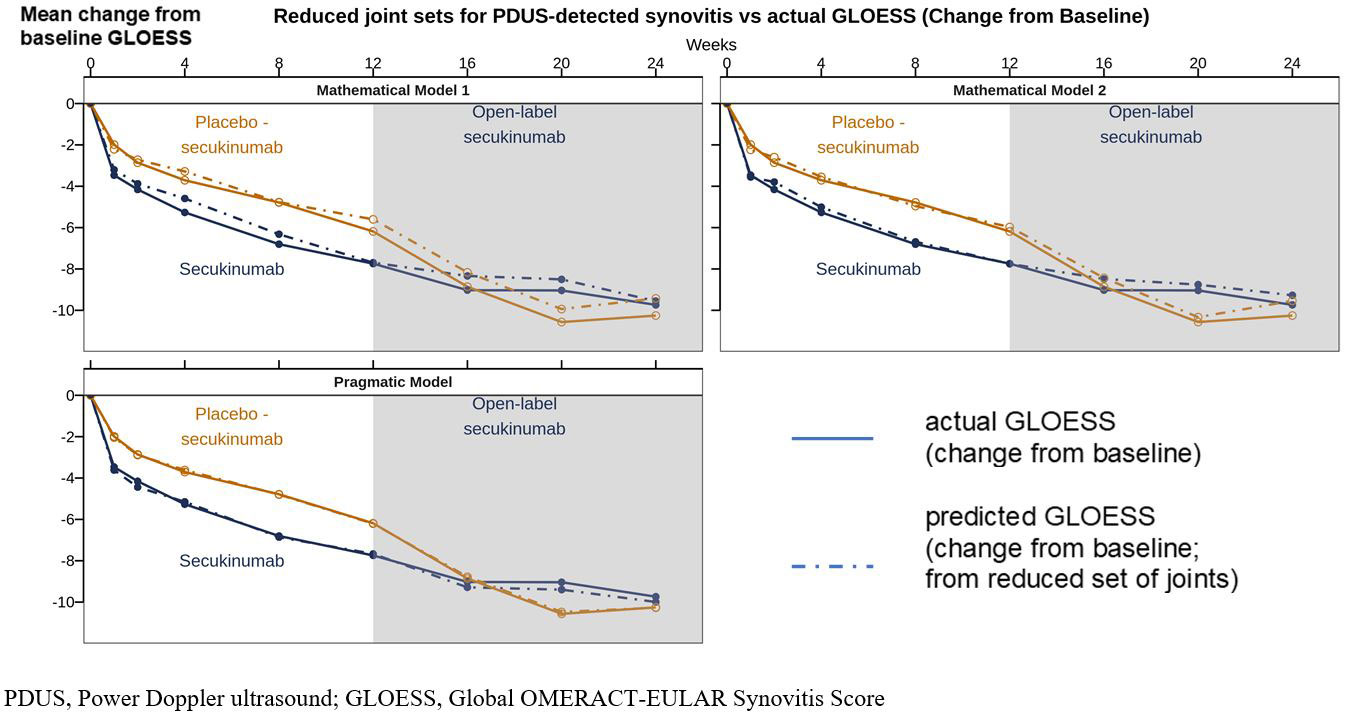
Results: Three models were established with reduced joint sets (12–18 pairs). The joints included in each linear model are summarized in Table 1. Figure 1 depicts the trajectory of the two mathematical models and the pragmatic model versus the actual GLOESS in the secukinumab and the placebo-secukinumab groups assessed by change from baseline to Week 24. The reduced PDUS joint sets demonstrated similar changes from baseline to Week 24 as the full GLOESS score.
Conclusion: The 3-ultrasound reduced joint sets demonstrated similar responsiveness to secukinumab as the full GLOESS score. Further studies are needed to confirm the validity and utility of the reduced joint set ultrasound scores in other PsA cohorts.
Reference
1. D’Agostino MA, et al. Rheumatology (Oxford) 2021;keab628
To cite this abstract in AMA style:
D'Agostino M, Conaghan P, Gaillez C, Naredo E, mandl P, Carron P, Šenolt L, Rosa J, Lopez Rdz A, Goyanka P, Sahoo B, Bao W, Schett G, Boers M. How Well Does Ultrasound-assessed Synovitis in Reduced Joint Sets Predict the Response to Secukinumab in Patients with Active Psoriatic Arthritis and Inadequate Response to Conventional DMARDs? – Exploratory Results from a Phase 3b Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/how-well-does-ultrasound-assessed-synovitis-in-reduced-joint-sets-predict-the-response-to-secukinumab-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-conventional-dmards-expl/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-well-does-ultrasound-assessed-synovitis-in-reduced-joint-sets-predict-the-response-to-secukinumab-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-conventional-dmards-expl/