Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Anti-nuclear autoantibodies are a hallmark of scleroderma with anti-centromere antibody (ACA) recognizing centromeric antigens. ACA-positive patients have longstanding Raynaud’s, limited cutaneous disease and increased risk for pulmonary arterial hypertension. We investigated the role of HLA classical genes and alleles on risk for ACA-positive scleroderma in a large collection of patients with scleroderma and genetically matched controls.
Methods: SNP genotypes of 723 scleroderma cases and 5,561 controls, all of European ancestry, were obtained from dbGaP. Classical HLA types were imputed with SNP2HLA using the Type I Diabetes Genetic Consortium reference of 5,225 individuals. Association of HLA classical alleles was tested by a dominant model regression analysis coding the HLA types as numeric values (1 for present, 0 for absent). Regression tests were corrected for genetic dissimilarity by including the top 5 principal components as covariates.
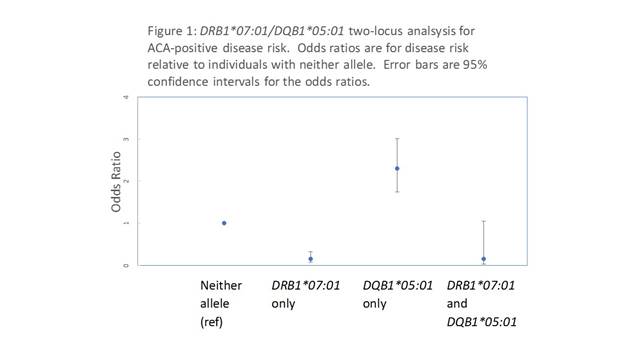
Results: Of the 723 scleroderma cases, 238 (32.9%) were positive for ACA. The most significantly ACA-positive scleroderma-associated HLA allele was HLA-DRB1*07:01, which was disease protective (P-value=1.8×10-18, odds ratio (OR)=0.11 (95%CI=0.05 to 0.22)). This allele was found in only 3.4% of the ACA-positive cases versus 23.6% of controls and 20.8% of the ACA-negative cases. Regression analysis conditioning on the disease-associated alleles identified HLA-DQB1*05:01 as the most significantly associated disease risk allele (P-value= 3.3×10-08, OR=2.18 (1.66-2.86)) with additional independent risk effects of HLA-DQA1*04:01 and HLA-DQA1*03:01). A two-locus analysis of the DQB1*05:01 disease-risk and the DRB1*07:01 disease-protective alleles suggested the risk effect of DQB1*05:01 is overridden by the protective effect of DRB1*07:01 (Figure 1). The odds ratio for ACA-positive disease in individuals carrying both alleles was 0.14, similar to that in individuals carrying DRB1*07:01 without DQB1*05:01 (0.15). No effect of DRB1*07:01 or DQB1*05:01 was found in the anti-topoisomerase I autoantibody subset (P=0.98 and P=0.054, respectively). For validation, 62 African American ACA-positive cases and 946 matched controls from the Genomic Research in African American Scleroderma Patients (GRASP) Collection, were similarly analyzed. DQB1*05:01 was associated with disease risk (P=3.6×10-4, OR=2.68 (1.57-4.58)) and DRB1*07:01 was protective (P=8.6×10-3, OR=0.33 (0.13-0.85)) in the African American sample.
Conclusion: HLA-DQB1*05:01 is associated with risk and HLA-DRB1*07:01 is associated with protection for ACA-positive scleroderma. The mechanisms responsible for these effects could be exploited to prevent or treat scleroderma.
To cite this abstract in AMA style:
Remmers EF, Alexander T, Morgan ND, Shah AA, Mayes MD, Adeyemo A, Doumatey A, Bentley A, Shriner D, Chandrasekharappa SC, Carns MA, Chung L, Criswell LA, Derk CT, Domsic RT, Gladue H, Goldberg A, Gordon JK, Hsu V, Jan R, Khanna D, Medsger TA Jr., Ramos PS, Trojanowski MA, Saketkoo LA, Schiopu E, Shanmugam V, Korman BD, Kron B, Bridges SL Jr., Kolstad KD, Bernstein EJ, Kafaja S, Maksimowicz-McKinnon K, Silver R, Steen VD, Varga J, Rotimi C, Boin F, Wigley FM, Kastner DL, Gourh P. HLA Contributions to Risk and Protection for Anti-Centromere Autoantibody-Positive Scleroderma [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/hla-contributions-to-risk-and-protection-for-anti-centromere-autoantibody-positive-scleroderma/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hla-contributions-to-risk-and-protection-for-anti-centromere-autoantibody-positive-scleroderma/