Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) regularly affects women of childbearing age1. A higher obstetric morbidity in women with RA is suggested in the literature in several countries, but no comparison between patients with RA and women in the general population is available for France. The aim of the study was to compare adverse fetal, maternal and pregnancy outcomes in women with RA with matched controls from the French general population.
Methods: We conducted a matched comparative study of RA patients included in the GR2 (Groupe de Recherche sur la Grossesse et les Maladies Rares) national multicentre cohort from 2014 to June 2021 and controls from the French general population included in the French national perinatal surveys (Enquête Nationale Périnatale)2. The latter is a national survey carried out for one week every 5 years and recording around 13 000 births. As births before 22 gestational week (GW) and birth weights < 500 grams were excluded from the French national perinatal survey, the same exclusion criteria were applied to women with RA from the GR2 cohort. Each pregnancy in patients with RA was matched to 4 pregnancies in controls on age group (≤ 29, 30-34, 35-39, or ≥ 40 years), parity, area of residence, and gemellity. These matching variables were chosen according to their association with adverse pregnancy outcomes (potential confounders). Births in the GR2 cohort between 2015 and 2018 were matched to births in the 2016 survey and those between 2019 and 2021 were matched to births in the 2021 survey. RStudio’s MatchIt package was used to match RA patients to controls. The frequency of each adverse pregnancy outcome was compared between the two groups using Fisher’s exact test or Chi-2. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated for each variable.
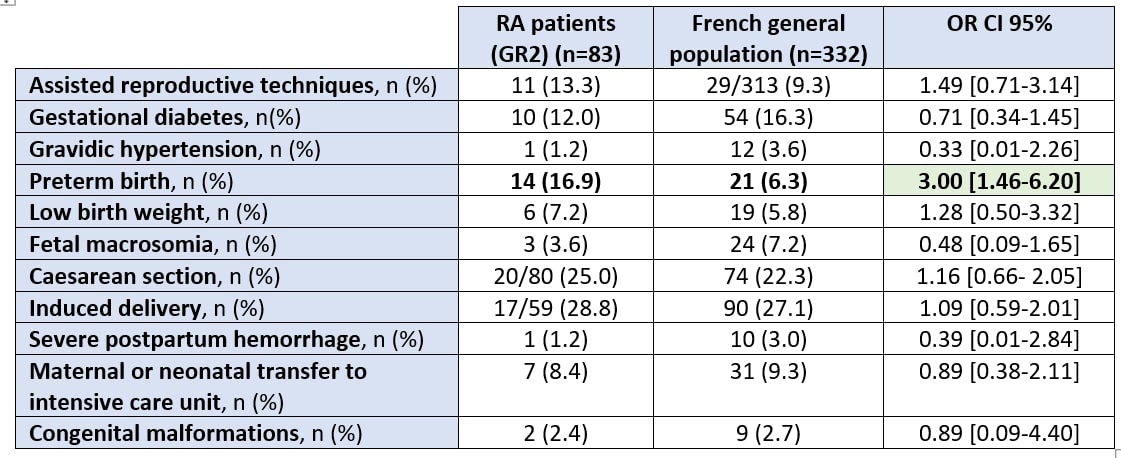
Results: Of the 92 patients with RA included in the GR2 cohort, 83 (including one twin pregnancy) were retained after excluding births before 22 GW and/or birth weight < 500 grams. They were matched with 332 control pregnancies from the French national perinatal survey (180 pregnancies from the 2016 survey and 152 from the 2021 survey). The mean age of RA patients was 33.7 (+/- 5.06) years and 53% were nulliparous. Preterm birth was significantly higher in newborns of RA patients than in newborns of controls (16.9% versus 6.3%, p < 0.01). Among the cases of preterm birth, only one case of extreme prematurity (at 30 GW) and no case of very extreme prematurity were found in the GR2 cohort. However, there was no significant difference for gestational diabetes nor macrosomia (despite the use of corticosteroids at least once during pregnancy in 42.1% of RA patients), gestational hypertension, low birth weight, severe postpartum hemorrhage, maternal or neonatal transfer to intensive care unit or congenital malformation between the two populations (Table 1).
Conclusion: This study found an increased risk of preterm delivery in RA patients compared with control women in the French general population, which is consistent with an increased risk observed in other countries. These results raise the need for particular attention to obstetrical follow-up in these patients and providing useful insights for physician-patient dialogue.
To cite this abstract in AMA style:
Hamroun S, Martin De Frémont G, Costedoat-chalumeau N, couderc M, Flipo R, Gossec L, Richez C, Belkhir R, Frazier A, Devauchelle V, Marotte H, Sellam J, Gervais E, Deroux A, Lukas C, Dernis E, Chatelus E, Le Guern V, Guettrot-Imbert G, Lelong N, Le Ray C, Seror R, Molto A. Higher Risk of Preterm Delivery in Women with Rheumatoid Arthritis: A Matched Comparative Analysis of the GR2 Prospective Cohort and the French National Perinatal Surveys [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/higher-risk-of-preterm-delivery-in-women-with-rheumatoid-arthritis-a-matched-comparative-analysis-of-the-gr2-prospective-cohort-and-the-french-national-perinatal-surveys/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/higher-risk-of-preterm-delivery-in-women-with-rheumatoid-arthritis-a-matched-comparative-analysis-of-the-gr2-prospective-cohort-and-the-french-national-perinatal-surveys/