Session Information
Date: Tuesday, November 9, 2021
Title: Reproductive Issues in Rheumatic Disorders Poster (1711–1731)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Pregnant women with SLE are at substantial risk of preeclampsia. Best international practice guidelines recommend aspirin (ASA) in this population, as it reduces preeclampsia risk by more than 50% in high-risk women. However, recent evidence suggests that only 25% of pregnant SLE women use ASA. It is therefore imperative to understand ASA use patterns in SLE pregnancies to promote ASA adherence. With this objective, we are conducting the PREPARE (PREeclamPsia knowledge & Aspirin adheRence in lupus prEgnancies) trial, a randomized controlled trial, evaluating a specifically-designed educational tool. In this analysis, we aimed to assess ASA use, adherence, and dosage at baseline and 2nd study visits according to the intervention status.
Methods: Since 2018, we are recruiting consecutive pregnant SLE women (diagnosed according to the SLICC criteria) until the 16th gestational week at 5 Canadian centers. Participants are randomly assigned to receive the educational tool (intervention) or standard of care (control). At baseline (i.e. 1st trimester) and 2nd visits (i.e. 2nd trimester, 20-24 weeks, for ongoing pregnancies or 4-8 weeks after end of pregnancy for women who miscarried), the participants complete self-reported ASA adherence questionnaires and the modified Adherence to Refills and Medications Scale (ARMS), scored out of 44 (lower score meaning better adherence). We defined the participants ‘ASA users’ if they were using ASA in ongoing pregnancies or if they had used ASA but had a miscarriage. Current analysis includes participants enrolled at the coordinating center (accounting for nearly half of the total planned sample size). We measured the proportion of ASA users, mean ARMS scores, and dosage at both visits. We estimated a 95% CI for difference in proportion of ASA users between the groups using the Wilson procedure and mean ARMS score difference between the groups using the Student’s t test.
Results: Thirty-three participants were included, 16 exposed to the intervention and 17 controls. Baseline characteristics were well-balanced with mean age of 32.2 years (standard deviation, SD, 4.6) in the intervention and 34.1 years (SD, 4.2) in the control group, and an identical proportion of participants who had post-secondary education (i.e. 80%) (Table 1). Baseline mean gestational age was 61.6 days (SD, 19.4) and 61.2 days (SD, 21.4) and baseline ASA use prevalence was 56% and 41% in the intervention and control group, respectively. Proportion of ASA users at the 2nd visit was 100% in the intervention and 82% in the control group, with a difference of 18% (95% CI -5, 41). At the 2nd visit, mean ARMS score was not different in the intervention (12.4 points) and control (12.2 points) groups [difference of 0.3 points (95% CI -0.8, 1.3)]. Among ASA users at the 2nd visit, 6% and 14% used 80-81mg, and 75% and 71% used 160-162mg in the intervention and control group, respectively.
Conclusion: Halfway into the trial, we observed a trend for higher ASA use in pregnant SLE women who received a specifically-designed educational tool compared to those receiving standard of care. The PREPARE trial is on track to provide a new evidence-based approach to optimize aspirin use and potentially improve outcomes in this population.
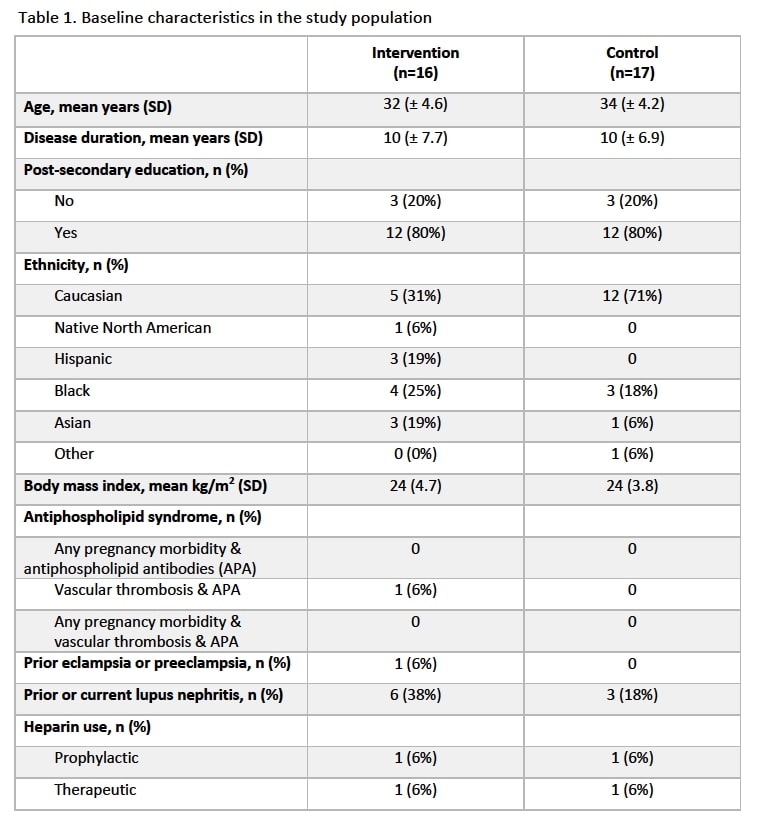 Table 1. Baseline characteristics in the study population.
Table 1. Baseline characteristics in the study population.
To cite this abstract in AMA style:
Lee J, Mendel A, Malhamé I, Bernatsky S, Vinet E. Higher Prevalence of Aspirin Use with a Specific Educational Tool in SLE Pregnancies: Preliminary Results [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/higher-prevalence-of-aspirin-use-with-a-specific-educational-tool-in-sle-pregnancies-preliminary-results/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/higher-prevalence-of-aspirin-use-with-a-specific-educational-tool-in-sle-pregnancies-preliminary-results/
