Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Previous studies have demonstrated that the risk of herpes zoster (HZ) among adults with rheumatic disease, such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), and systemic lupus erythematosus (SLE), is higher when compared to the immunocompetent US adult population aged 50 years and older. However, while earlier estimates provided valuable guidance on HZ risk, a gap remains related to the association of this risk with both the underlying condition and use of immunosuppressive (IS) medication.
Methods: This was a retrospective cohort study of commercial and Medicare Advantage with Part D health plan members using October 2015-December 2022 administrative claims data. Adults ≥18 years old with ≥1 claim for an IS medication and continuous enrollment for ≥12 months prior to the first IS medication fill (index date) were eligible. Patients with ≥1 claim for a diagnosis of RA (ICD-10: M05.x, M06.x), PsA (ICD-10: L40.5x), or SLE (ICD-10: M32.x) were then identified by diagnostic codes. Patients were followed until the earlier of an HZ diagnosis or vaccination, pregnancy, end of enrollment or study period, or death. HZ incidence rates (IR) per 1,000 person-years were calculated for each rheumatic disease and multivariable Cox proportional hazard models produced adjusted hazard ratios (aHRs) that controlled for baseline characteristics and time-varying IS status during follow-up.
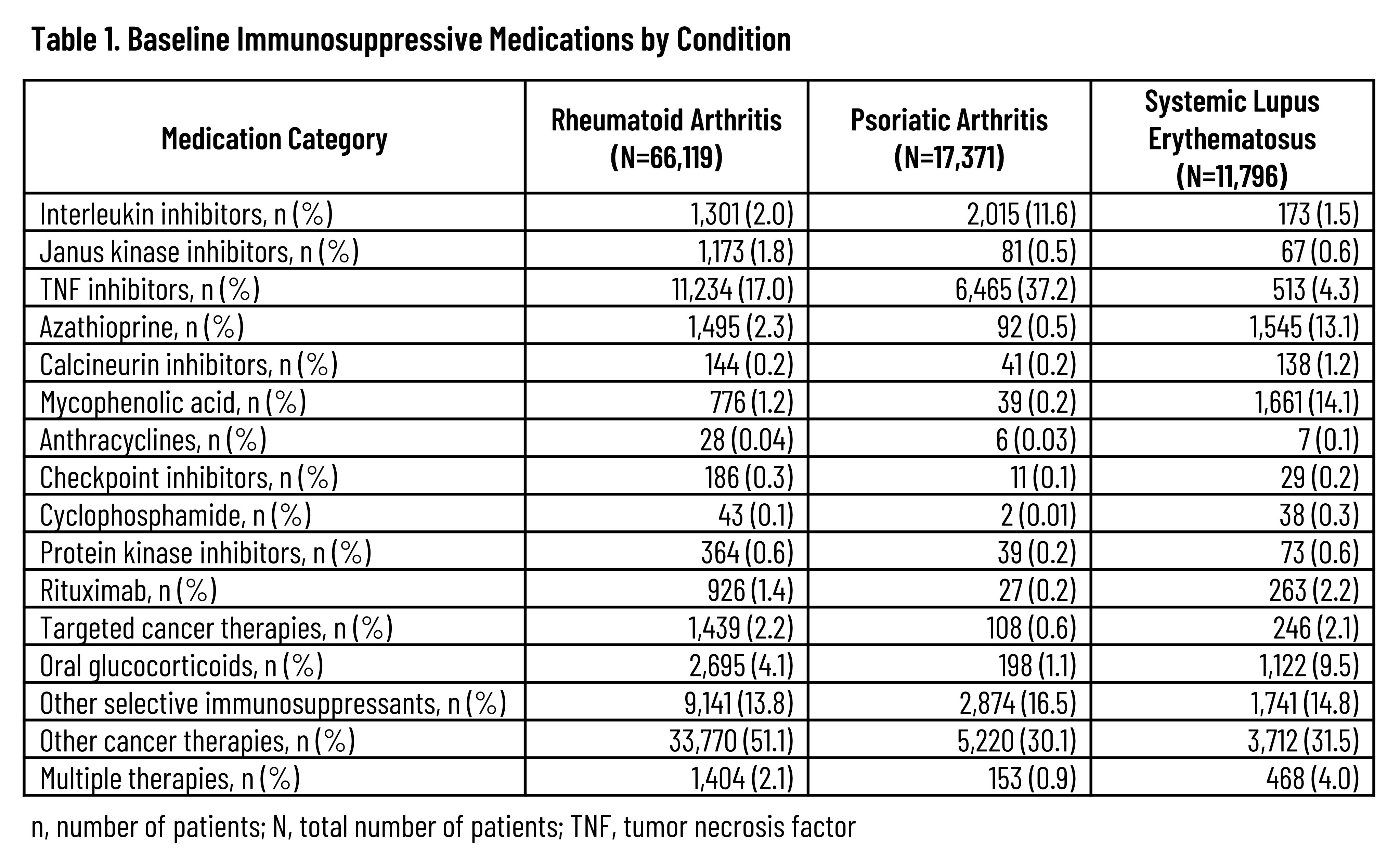
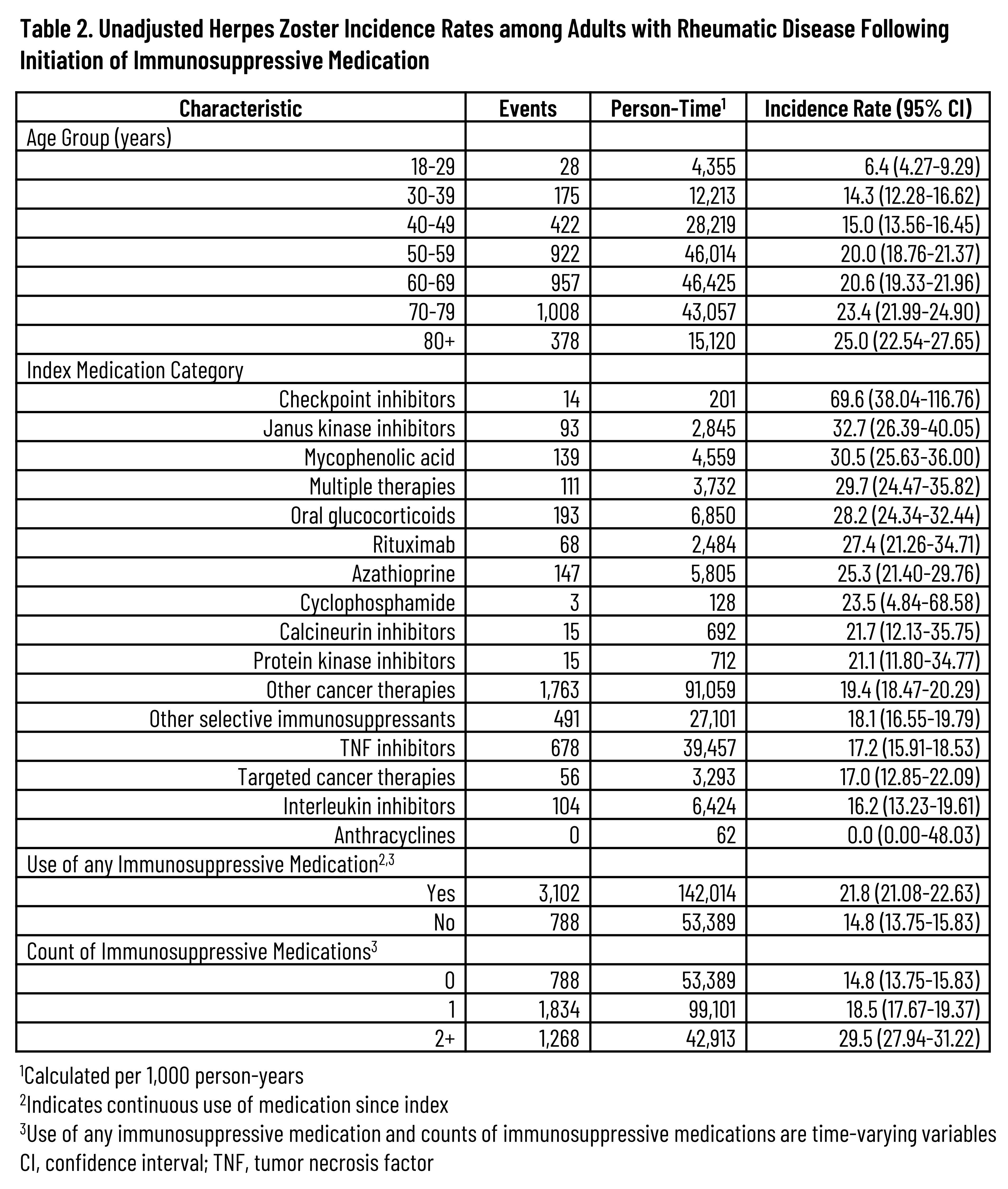
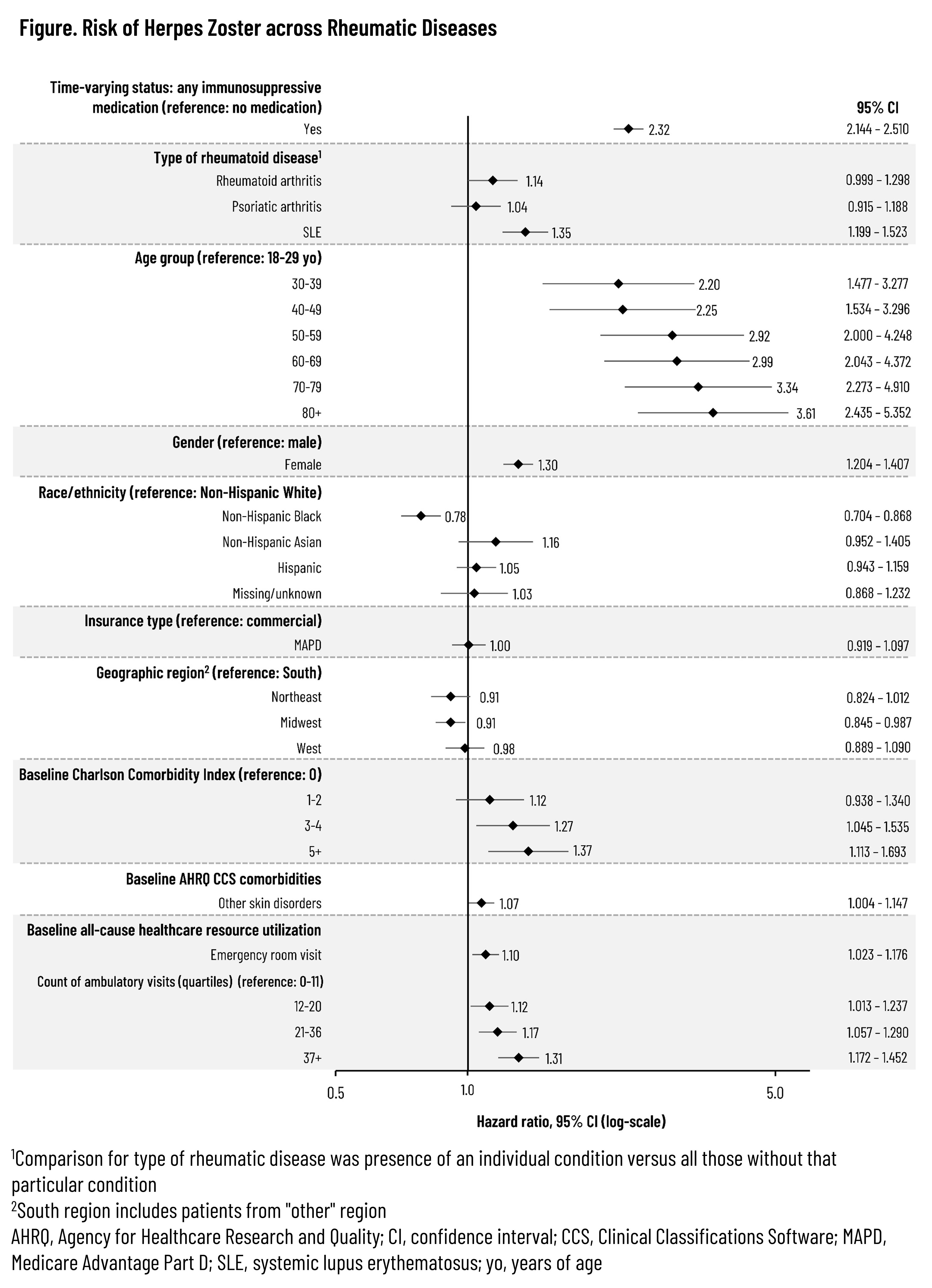
Results: Across the 3 rheumatic diseases, 87,372 unique patients were followed for a median of 648 days (interquartile range: 263-1,319 days). The distribution of IS therapies across each disease is listed in Table 1. A total of 4,295 HZ events were observed, resulting in an overall unadjusted HZ IR of 19.9 (95% confidence interval [CI]: 19.29-20.54) that tended to increase with age and the number of IS medication classes while also varying by index medication class (Table 2). Unadjusted IRs by condition were 20.8 (95% CI: 20.11-21.57), 15.5 (95% CI: 14.25-16.77), and 24.0 (95% CI: 22.12-26.09) for RA, PsA, and SLE, respectively. Across all patients with the included conditions, the aHR was 2.32 (95% CI: 2.144-2.510) based on time-varying use of any (vs. no) IS medication (Figure). Adjusted hazards tended to increase with older age and higher comorbidity index and were also higher among females but lower for non-Hispanic Black adults vs non-Hispanic White adults. Compared to patients with RA or PsA, those with SLE had an aHR of 1.35 (95% CI: 1.199-1.523). Among those with sufficient follow-up, 9.9% had herpes zoster ophthalmicus and 21.1% developed post-herpetic neuralgia following the HZ event.
Conclusion: Adults with rheumatic disease are at elevated risk of HZ following the initiation of IS medications, and patients with SLE initiating IS medication appear to be at a higher risk compared to patients with RA or PsA. Such risk reinforces the need for the entire rheumatology care team to consider the importance of HZ prevention when IS therapy is being considered, irrespective of patient age.
To cite this abstract in AMA style:
Gatwood J, Zhu Y, Steffens A, Gallagher S, DuCharme M, Stempniewicz N. Herpes Zoster Risk Following Initiation of Immunosuppressive Therapy Among Adults with Rheumatic Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/herpes-zoster-risk-following-initiation-of-immunosuppressive-therapy-among-adults-with-rheumatic-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/herpes-zoster-risk-following-initiation-of-immunosuppressive-therapy-among-adults-with-rheumatic-disease/