Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Structural differences exist between the healthcare systems in the United States (US) and Europe. In real-world clinical settings, we investigated whether these differences create inequalities between the two regions in the standard of care for patients with axial spondyloarthritis (axSpA). Identifying differences will be the first step to targeted interventions in each region, enabling health equity.
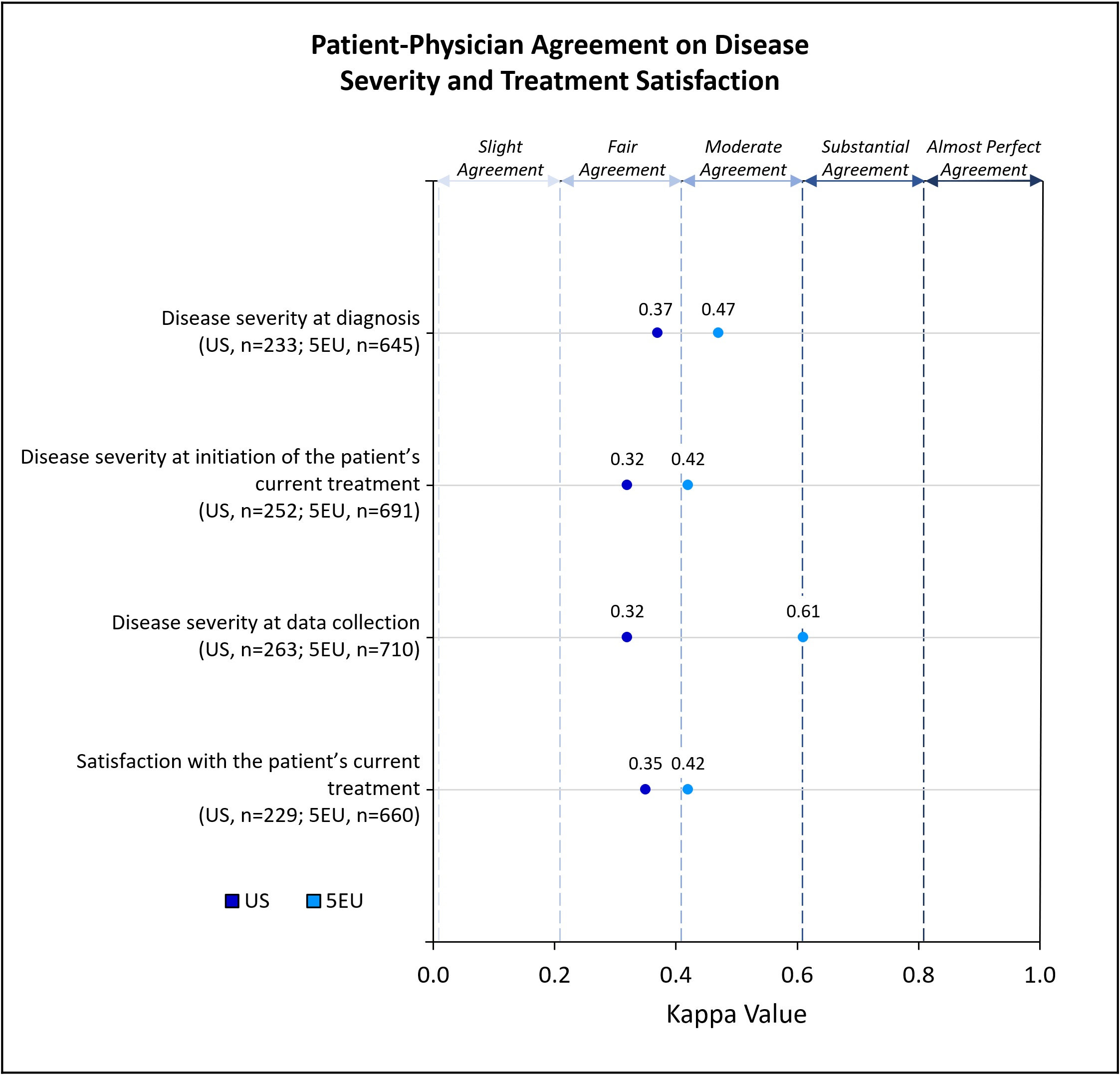
Methods: Data were drawn from the Adelphi Real World axSpA Disease Specific Programme™, a cross-sectional survey, with retrospective data collection, of rheumatologists and their consulting patients with axSpA. The survey was conducted from March – November 2021 (“data collection”) in 5EU (France, Germany, Italy, Spain, United Kingdom) and the US. Weighted (linear) kappa analyses measured agreement where patients and their rheumatologists reported perceptions of the patient’s disease severity at time of survey and retrospectively (at diagnosis, at current treatment initiation), and satisfaction with the patient’s current treatment. Kappa value interpretation ranges from no agreement (≤0) to almost perfect agreement (0.81-1). Differences between the US and 5EU where physicians reported details of demographics, symptomology, hospitalizations, and consultations were measured using Chi-square, Fisher’s exact and t-tests.
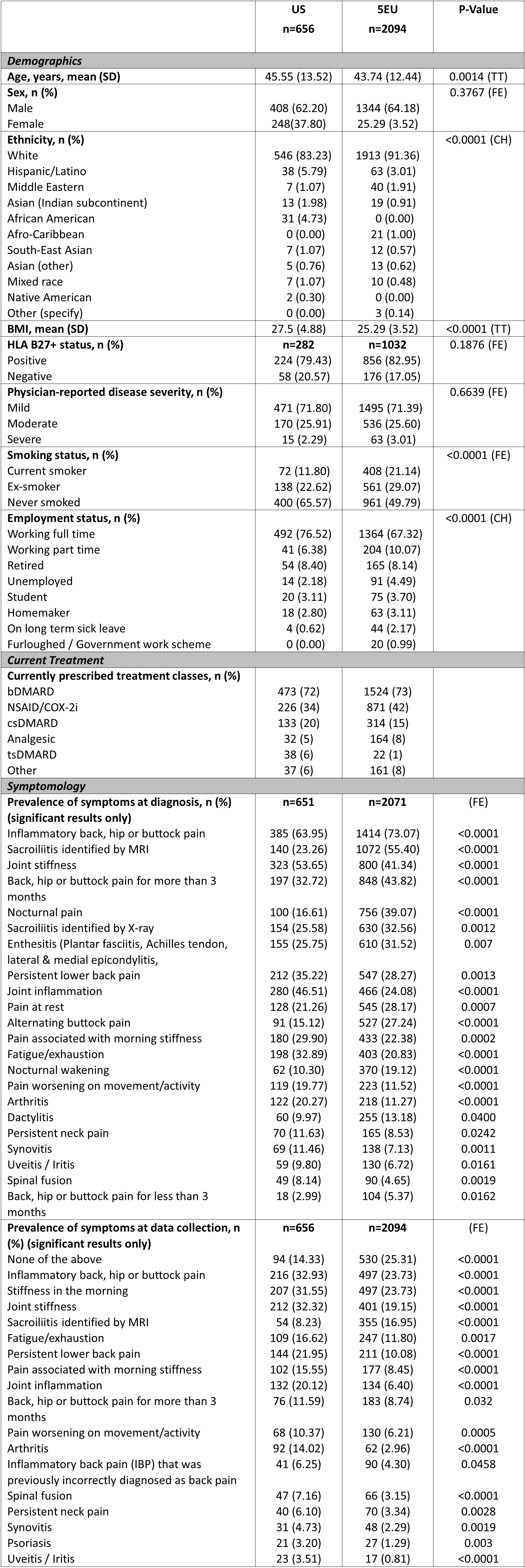
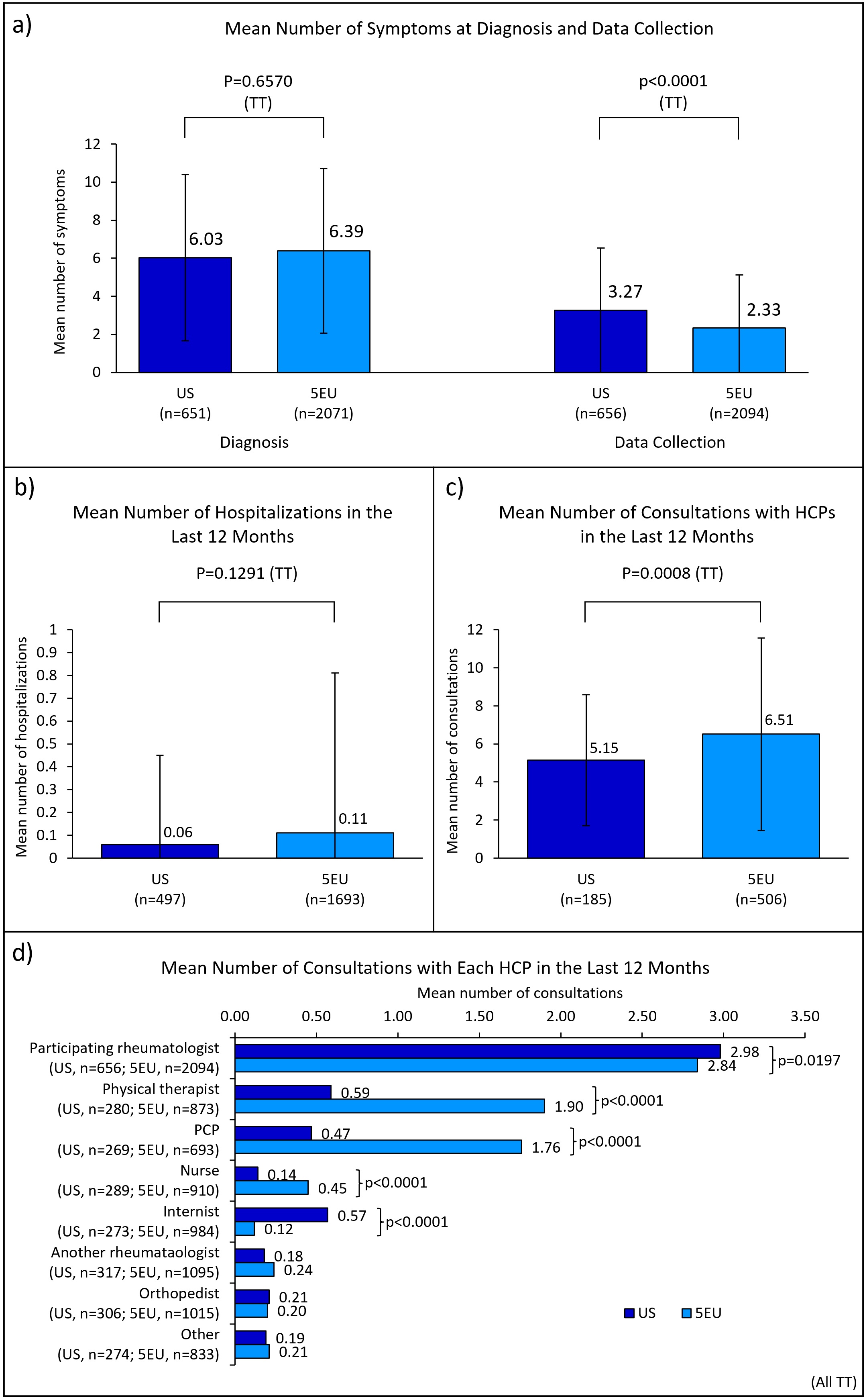
Results: Rheumatologists (n=334; US=81, 5EU=253) provided data for 2750 patients (US=656, 5EU=2094). Some demographics significantly differed between regions; nearly ¾ of patients in the US (72%) and 5EU (73%) were prescribed a biologic disease-modifying antirheumatic drug (bDMARD) (Table 1). Patient-physician agreement on perceived axSpA disease severity was greater in 5EU than the US, at diagnosis (moderate vs fair), initiation of current treatment (moderate vs fair) and at data collection (substantial vs fair); agreement on treatment satisfaction was greater in 5EU than the US (moderate vs fair) (Figure 1). While the mean [standard deviation (SD)] number of symptoms present at diagnosis was similar between the US and 5EU (6.03 [4.38] vs 6.39 [4.33]; p=0.0657), the mean [SD] at data collection was significantly greater in the US than 5EU (3.27 [3.25] vs 2.33 [2.84]; p< 0.0001) (Figure 2). Prevalence of several individual symptoms also varied by region (Table 1). Mean [SD] number of consultations with healthcare professionals (HCPs) in the previous 12 months was significantly lower among US patients than 5EU patients (5.15 [3.44] vs 6.51 [5.06]; p=0.008); mean number of consultations with individual HCP types also differed significantly (Figure 2). No significant difference was found between the mean number of hospitalizations in the two regions (Figure 2).
Conclusion: US axSpA patients experienced greater discordance with their physicians on disease severity and treatment satisfaction and experienced significantly more symptoms vs 5EU patients at data collection. Number of consultations appeared to be greater in 5EU than the US. The reasons for these inequalities will be complex and better understanding why these differences exist will be key to implementing targeted improvements in both regions.
To cite this abstract in AMA style:
Nikiphorou E, Ogdie A, Twigg D, Quiñones E, Ling Y, Masri K, Cappelleri J, Sanchez-Riera L, Hughes M, MASSEY N. Health Inequalities Exist Between the United States and Europe for Patients with Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/health-inequalities-exist-between-the-united-states-and-europe-for-patients-with-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/health-inequalities-exist-between-the-united-states-and-europe-for-patients-with-axial-spondyloarthritis/