Session Information
Title: Rheumatoid Arthritis - Clinical Aspects II: Identifying Rheumatoid Arthritis in At-Risk Populations
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Multiple studies demonstrate that there is a preclinical period of rheumatoid arthritis (RA) when autoantibodies (Abs) including RF and anti-CCP are abnormal in absence of clinically apparent inflammatory arthritis (IA). Furthermore, RF and CCP abnormalities are highly predictive of future classifiable RA. However, most data regarding preclinical RA and prediction of future disease are derived from retrospective studies or referral clinics for symptomatic patients, and little is known from prospective community-based studies. Therefore, in a community health fair we identified and followed subjects with abnormalities of RA-related Abs in the absence of IA to evaluate predictive factors for development of IA/RA.
Methods:
Volunteers were tested for serum anti-CCP3 (INOVA) at a Colorado health fair from 2008-2012. Subjects with CCP3 positivity (≥20 units) without IA by physical examination were invited for biannual follow-up that included assessment of demographic factors, environmental exposures, joint symptoms, joint examination by a trained study physician or nurse, and testing of CCP3 and CCP3.1 (INOVA), CCP2 (Axis-Shield), RF by nephelometry (Dade-Behring) and specific isotypes IgG/M/A (INOVA), C-reactive protein (CRP), and the shared eptiope (SE). The primary outcomes were the development of IA (≥1 swollen joint), and RA by 1987 and/or 2010 criteria at a research visit or clinical evaluation by a rheumatologist outside of the study.
Results:
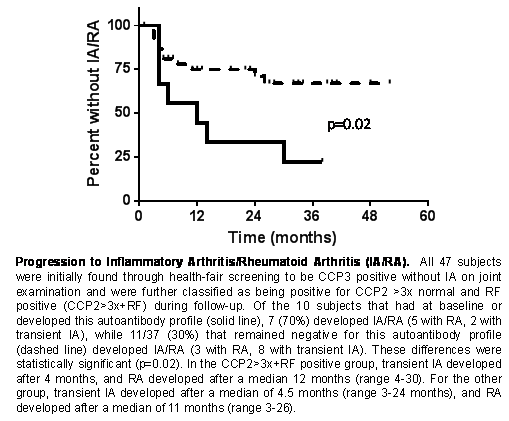
7178 volunteers were initially evaluated, and 158 (2%) were CCP3(+) without IA; 47 of 158 (30%) invited subjects agreed to further study and were followed for a median of 27 months (range 1-52). Of these, 18/47 (38%) developed IA or RA all within 36 months: 10 with transient IA defined as ≥1 swollen joint(s) not present at an evaluation ~6 weeks later, and 8 with RA (7 by 1987 and 8 by 2010 criteria). The factor most strongly associated with the development of either IA or RA was CCP2 >3x normal plus positivity for any RF assay at any level (OR 5.5, 95% CI 1.2-25.3; sensitivity [SENS] 39%, specificity [SPEC] 90%, positive predictive value [PPV] 70%). This same Ab profile exhibited a stronger association with development of classified RA (OR 11.3, 95% CI 2.0-62.8; SENS 63%, SPEC 87%; PPV 50%). The timing of development of IA or RA is presented in the Figure. Age, sex, smoking, family history of RA, baseline joint symptoms, CRP and SE were not significantly associated with developing IA/RA.
Conclusion:
A health fair evaluation using initial CCP3 testing is able to identify subjects at-risk for future IA or RA. In particular, during follow-up, CCP2 >3x normal and RF positivity were most strongly associated with development of IA or RA. This method of identifying subjects at risk for RA is useful for understanding the natural history of disease development, and may ultimately be used to identify candidates for RA prevention.
Disclosure:
K. E. Beck,
None;
M. K. Demoruelle,
None;
C. C. Striebich,
None;
K. Aiona,
None;
M. L. Feser,
None;
L. A. Derber,
None;
S. Brake,
None;
J. Lysengen,
None;
L. Rosseisen,
None;
J. J. Rhiannon,
None;
S. M. Weisman,
None;
J. M. Norris,
None;
V. M. Holers,
None;
K. D. Deane,
AbbVie,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/health-fair-evaluations-to-identify-subjects-at-risk-for-future-inflammatory-arthritis-and-rheumatoid-arthritis/