Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders III: Clinical Trials
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Tocilizumab (TCZ), rituximab (RTX), mycophenolate mofetil (MMF), and cyclophosphamide (CYC) are the immunosuppressants (IS) with the current best evidence for the treatment of systemic sclerosis-associated interstitial lung disease (SSc-ILD)1-4. However, limited information is available from comparison studies, and these treatments have often been tested in diverse populations. This study aimed to compare the effectiveness of TCZ, RTX, MMF, and CYC in SSc-ILD patients from the EUSTAR database.
Methods: We included SSc patients from the EUSTAR database who fulfilled the following criteria: 1/ radiologically confirmed ILD; 2/ database record on the use of TCZ, RTX, MMF, or CYC; 3/ baseline and follow-up lung function associated with the treatment period. The primary endpoint was the change of forced vital capacity (FVC) % predicted from baseline to follow-up. Secondary endpoints included changes in diffusing capacity (DLCO) % predicted, modified Rodnan Skin Score (mRSS), and categorical changes in lung function tests and mRSS. Missing data were processed using multiple imputations with chained equations. Selection bias of the medications was reduced to a minimum using propensity score-based inverse probability of treatment weighting (IPTW). For paired therapy comparisons, we used average treatment effects (ATE). Subgroup analysis included previous IS use, background IS, and treatment duration (less than 18 months).
Results: 995 patients with 1050 treatment observations were included in this study. TCZ patients had more arthritis, higher baseline FVC and DLCO, more reticular change in chest CT, and less ground glass opacity. RTX and CYC patients had more elevations of C-reaction protein, and TCZ and RTX patients had higher baseline mRSS, more digital ulcers, and fewer IS naive cases (Table 1).
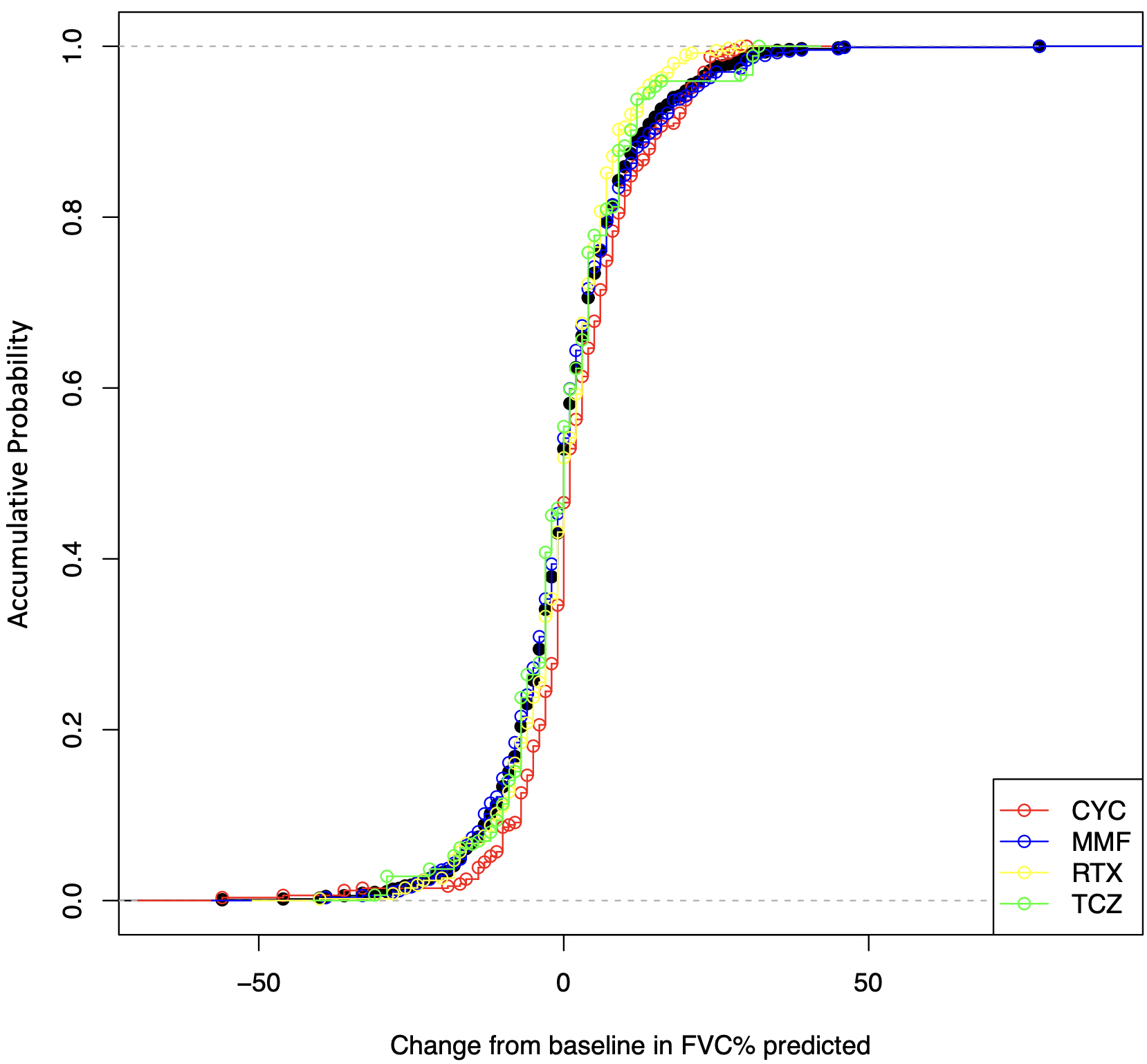
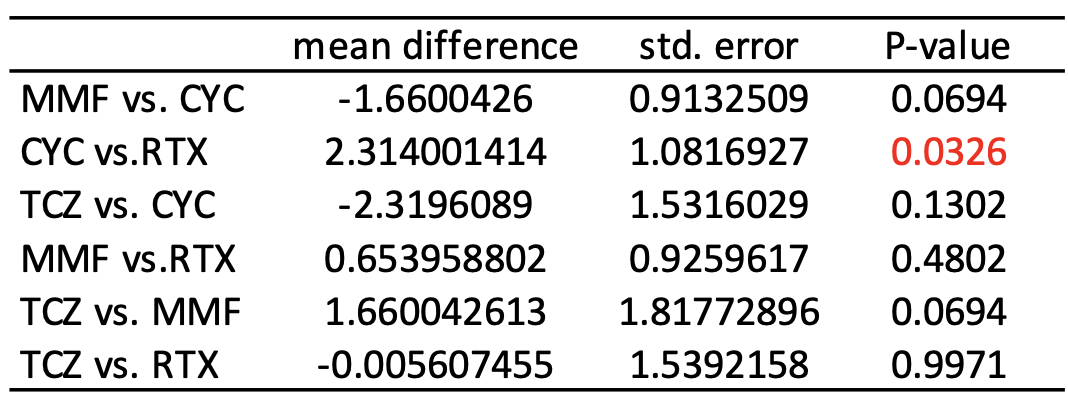
The median follow-up time was 11 months (interquartile range: 8-14 months). After IPTW, the changes of FVC % predicted were not significantly different between the four treatment arms by the Kruskal Wallis test (P=0.058, Figure 1). Among paired comparisons, only the treatment difference between CYC vs. RTX was significant (mean difference of FVC % predicted, 2.29; 95% CI, 0.18-4.40; P=0.034, Table 2).
In the multivariate logistic regression, only CYC was associated with stable or improved FVC among the four agents. The treatment differences in change of FVC% predicted among the four groups were not significant in patients with previous IS, background IS, or treatment duration > 18 months. Interestingly, MMF showed better FVC responses in patients with previous IS than in IS naive patients [Mean adjusted change of FVC % predicted (95% CI): 1.90 (0.36 to 3.45) vs. -0.04 (-1.35 to 1.26), P=0.047].
Conclusion: In this first large real-world observational study, the treatment effectiveness on FVC change of SSc-ILD patients was not statistically different between TCZ, RTX, MMF, and CYC. CYC showed clinically marginal but statistically significantly better effectiveness in some sub-analyses. Head-to-head randomized clinical trials on IS with longer follow-up are needed for SSc-ILD.
To cite this abstract in AMA style:
Yan Q, Bruni C, Garaiman A, Mihai C, Jordan S, Becker M, Elhai M, Dobrota R, Petelytska l, Hoffmann-Vold A, Henes J, Hachulla E, Siegert E, Balbir-Gurman A, Cuomo G, Riemekasten G, Heitmann S, Riccieri v, Ullman S, Sfikakis P, Ingegnoli F, Bernardino V, Truchetet M, Del Galdo F, YE S, Distler O. Head-To-Head Comparison of the Effectiveness of Tocilizumab, Rituximab, Mycophenolate Mofetil, and Cyclophosphamide in Patients with SSc-ILD from the EUSTAR Database [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/head-to-head-comparison-of-the-effectiveness-of-tocilizumab-rituximab-mycophenolate-mofetil-and-cyclophosphamide-in-patients-with-ssc-ild-from-the-eustar-database/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/head-to-head-comparison-of-the-effectiveness-of-tocilizumab-rituximab-mycophenolate-mofetil-and-cyclophosphamide-in-patients-with-ssc-ild-from-the-eustar-database/