Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Guselkumab (GUS), a targeted inhibitor of IL-23p19, demonstrated significant efficacy v placebo (PBO) in achieving ACR20 response at week (W) 24 in patients (pts) with active PsA in 2 Phase 3 trials, DISCOVER-1 & 2.1,2 Pts with PsA report pain relief as a priority for treatment.3 We conducted post hoc analyses to further assess GUS effect on pt-reported pain across outcome measures and maintenance of pain relief with up to 2 years (yr) of GUS.
Methods: DISCOVER-1 (1 yr) included 381 pts with active PsA despite standard therapies, including 1-2 TNF inhibitors in 31% of pts. DISCOVER-2 (2 yr) included 739 biologic-naïve pts with active PsA. In both studies, pts were randomized (1:1:1) to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or PBO with crossover to GUS 100 mg Q4W at W24 (PBO→Q4W). Pts rated pain using a 0-10 VAS (Pt Pain) as part of ACR, Disease Activity in Psoriatic Arthritis, and Minimal Disease Activity response criteria and reported Bodily Pain intensity over the past 4 W via the 36-Item Short-Form Health Survey (SF-36) question 21 (0-5). Pts with spondylitis and peripheral arthritis at baseline (BL) rated their Spinal and Joint Pain (0-10) as part of BASDAI. Pain data were evaluated for observed mean change, percent improvement from BL, and proportion of pts achieving ≥20% or ≥50% improvement in Pt Pain (nonresponder imputation [NRI]). Percent improvement from BL in tender (TJC; 0-68) and swollen (SJC; 0-66) joint counts, determined by independent assessors, was determined to evaluate consistency of improvements in pt-reported v physician-derived pain measures.
Results: In DISCOVER-2, mean BL Bodily Pain (range across groups: 4.4-4.5), Pt Pain (6.2-6.3), Spinal Pain (6.5-6.7), Joint Pain (6.3-6.8), SJC (11.7-12.9) & TJC (19.8-22.4) indicated moderate pain and disease activity at study outset. GUS-treated pts reported ~2x the improvement in Pt Pain, Spinal Pain, Joint Pain & Bodily Pain intensity at W24 v PBO; GUS improvements were maintained or further increased at W52 & W100. PBO→Q4W pts had similar improvements in pain as GUS-randomized pts (Table 1, Figure). Pt-reported pain appeared more sensitive to treatment effect, with larger differences in percent improvement v PBO, than physician-reported TJC/SJC at W24 (Figure); further research is warranted. While patterns were consistent, pain improved to a lesser degree than TJC/SJC, suggesting other causative factors. Consistent results were seen in DISCOVER-1 through 1 yr (data not shown). Among 748 GUS-treated pts across DISCOVER-1 & 2, substantial proportions achieved meaningful improvement in Pt Pain at early time points: 32% (W4) and 48% (W8) achieved ≥20% improvement; 28% (W12) and 33% (W16) achieved ≥50% improvement. At W24, 63% and 39% reported ≥20% and ≥50% pain improvement (Table 2).
Conclusion: GUS provided consistent and durable improvements in pt-reported pain across several measures. Pt-reported pain as an early and sensitive indicator of treatment effect in pts with active PsA and other factors underlying pain merit further evaluation.
References
1. Deodhar A. Lancet. 2020;395:1115-25
2. Mease P. Lancet. 2020;395:1126-36
3. Gudu T. Expert Rev Clin Immunol. 2018;14:405-17
 Table 1: Observed Mean (SD) Change from Baseline in Pain Scores, TJC, and SJC at W24, W52, and W100 in DISCOVER_2
Table 1: Observed Mean (SD) Change from Baseline in Pain Scores, TJC, and SJC at W24, W52, and W100 in DISCOVER_2
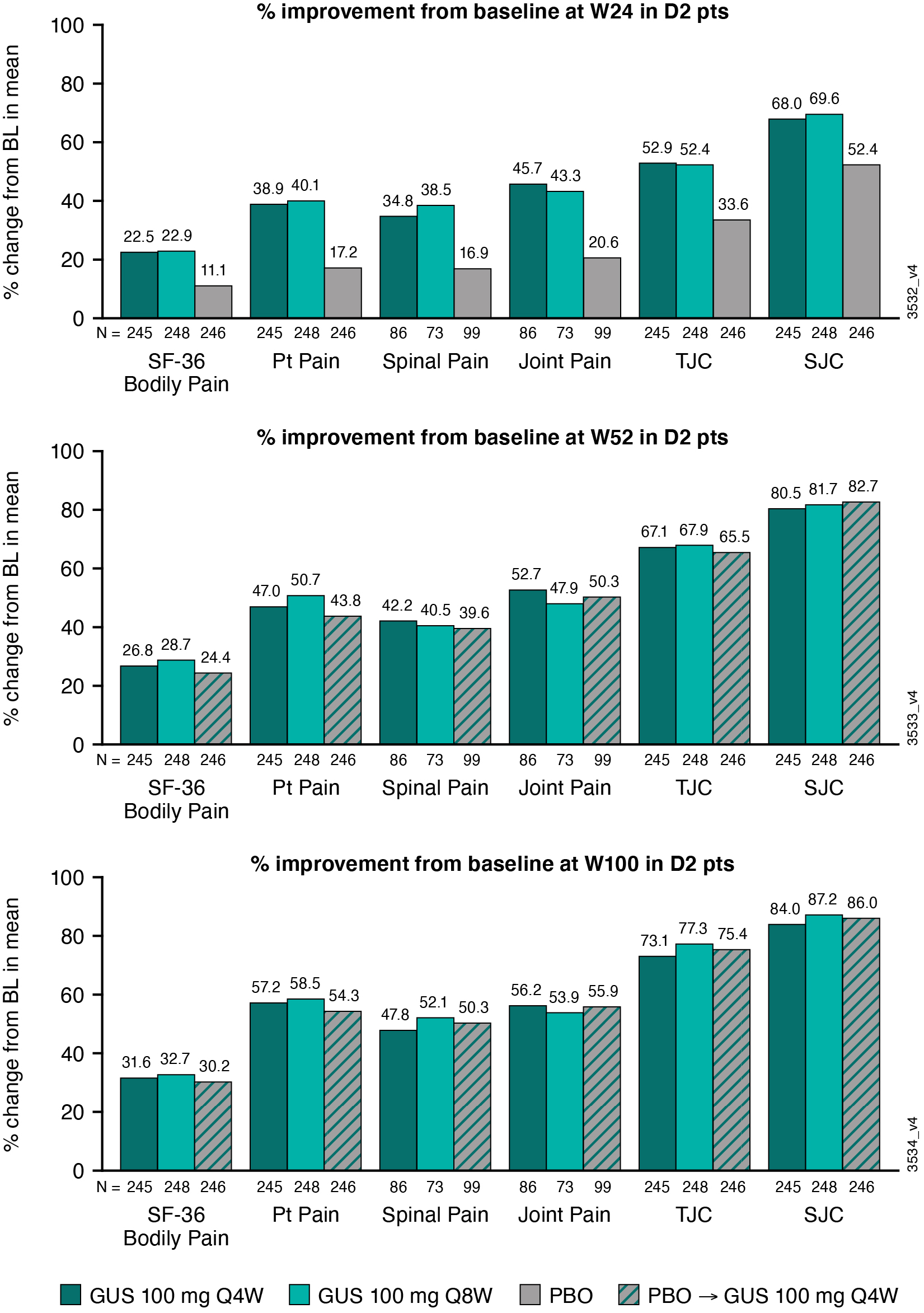 Figure: Percent Improvement from Baseline in Pt-Reported Pain Scores and Physician-Reported TJC and SJC in DISCOVER_2 Pts
Figure: Percent Improvement from Baseline in Pt-Reported Pain Scores and Physician-Reported TJC and SJC in DISCOVER_2 Pts
 Table 2: Proportion of Guselkumab (Q4W+Q8W) Randomized Pts Pooled Across DISCOVER_1 & 2 (Nf748) Reporting ≥20% or ≥50% Improvement in Pt Pain Through W24 (NRI)
Table 2: Proportion of Guselkumab (Q4W+Q8W) Randomized Pts Pooled Across DISCOVER_1 & 2 (Nf748) Reporting ≥20% or ≥50% Improvement in Pt Pain Through W24 (NRI)
To cite this abstract in AMA style:
Nash P, Tam L, Tsai W, Leung Y, Furtner D, Sheng S, Wang Y, Shawi M, Kollmeier A, Sherlock J, Cua D. Guselkumab (TREMFYA®) Provides Consistent and Durable Pain Improvement in Patients with Active Psoriatic Arthritis: Results of 2 Phase 3, Randomized, Controlled Clinical Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/guselkumab-tremfya-provides-consistent-and-durable-pain-improvement-in-patients-with-active-psoriatic-arthritis-results-of-2-phase-3-randomized-controlled-clinical-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-tremfya-provides-consistent-and-durable-pain-improvement-in-patients-with-active-psoriatic-arthritis-results-of-2-phase-3-randomized-controlled-clinical-trials/
