Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
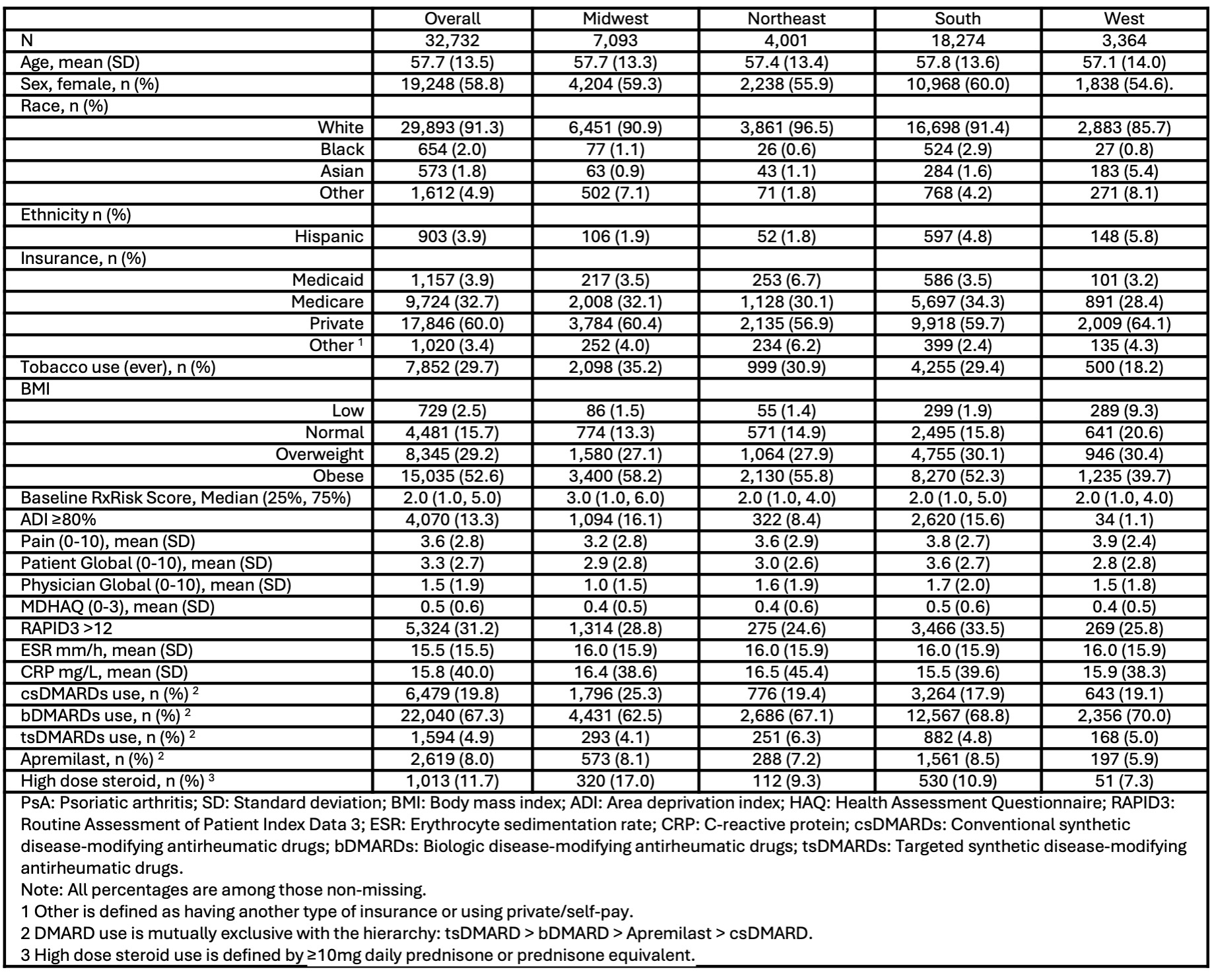
Background/Purpose: There is regional variation in the clinical characteristics, treatment, and outcomes of patients with inflammatory arthritis in the US. Despite a higher reported prevalence of PsA in the US, and an association with obesity and the metabolic syndrome, little is known about regional and ethnic variation of PsA disease activity, disease burden, and medication utilization in the US. We evaluated the distribution of PsA disease activity, medication use, insurance coverage, and comorbidity across US regions.
Methods: PsA patients from 198 practice sites in the ACR’s Rheumatology Informatics System for Effectiveness (RISE) EMR-based registry (1/2020-3/2023) with self-reported race and region of care comprised the study cohort. The distribution of clinical characteristics, disease activity and clinical measures [BMI, MDHAQ, pain, patient/physician global, ESR, CRP, high disease activity (HDA – RAPID3 >12)], high comorbidity [RxRisk >90th percentile], insurance coverage, and use of conventional, biologic, and targeted-synthetic DMARDs across geographic regions of the US were evaluated. Vulnerable patients were defined by Area Deprivation Index (ADI) 80. We used descriptive statistics anchored at the index date with an 18-month ascertainment window for demographic and clinical measures. Medication use reflected prednisone ((not mutually exclusive, with high dose defined as ≥ 10mg) and DMARD use (mutually exclusive) during the 18-month period prior to index. Regression models evaluated correlates of HDA and comorbidity, while adjusting for age, sex, race, ethnicity, and insurance.
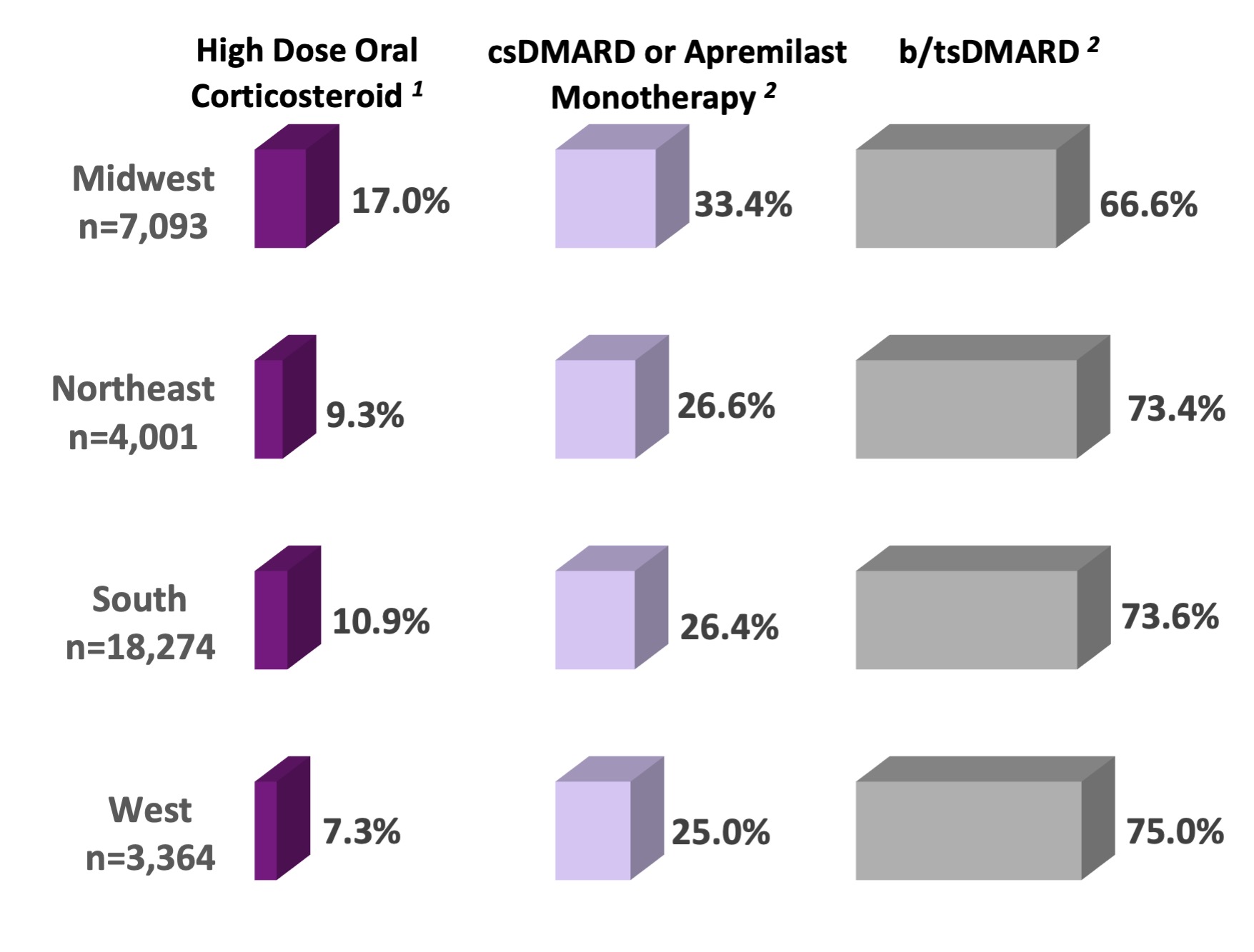
Results: Of 32,732 PsA RISE patients, most were White women with a mean (SD) age of 57.7(13.5) years and with private insurance (60%). Most patients (81.8%) had a BMI of > 25, and patients in the Midwest had the highest mean BMI (32.4 ±7.8). The median (25, 75) RxRisk was 2.0 (1.0, 5.0) and ADI ≥80 was present in 13.3%. Almost a third (31.2%) of patients had HDA, with the highest prevalence in the South (33.5%). There were regional variations in comorbidity and medication use. Approximately a quarter of the cohort (26%) received oral corticosteroids (OCS) and high dose OCS was more prevalent in the Midwest (17.0%), as was csDMARDs use compared to other regions (25.3%). Midwest PsA patients also reported more frequent use of high dose OCS use in combination with csDMARDs (31.3%) compared to other regions (NE 20.5%, South 13.6%, West 17.6%). In contrast, the lowest prevalence of bDMARD use was in the Midwest (62.5% vs. 67.1% NE, 68.8% South, and 70.0% West). Additionally, PsA patients in the Midwest had the highest odds of high comorbidity [OR: 1.73 (1.17-2.56, p=0.006].
Conclusion: Treatment of PsA disease varies across US regions with higher-than-expected use of high dose corticosteroids and in patients with multimorbidity and obesity. Further work is needed to understand the drivers behind treatment trends in these regions to optimize treatment and mitigate adverse outcomes in PsA patients.
1 High dose oral corticosteroid defined as ≥10mg daily prednisone or prednisone equivalent. High dose oral corticosteroid is the proportion of high dose steroid users among all patients who had steroid use (n=8,635; 26% of PsA cohort).
2 DMARD use is mutually exclusive with the hierarchy: tsDMARD > bDMARD > Apremilast > csDMARD monotherapy.
Figure. Regional Use of DMARDs and High Dose Corticosteroids in PsA Patients
(n=32,732) (%)
To cite this abstract in AMA style:
Banbury B, Dowell S, Jenkins C, Holladay E, Clinton C, Xie F, Zhang J, Wright G, Curtis J, Kerr G. Greater Glucocorticoid and Less Biologic/Targeted Therapy Use in Midwest PsA Patients Despite Prevalent Comorbidity [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/greater-glucocorticoid-and-less-biologic-targeted-therapy-use-in-midwest-psa-patients-despite-prevalent-comorbidity/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/greater-glucocorticoid-and-less-biologic-targeted-therapy-use-in-midwest-psa-patients-despite-prevalent-comorbidity/