Session Information
Date: Sunday, November 12, 2023
Title: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Initiating urate-lowering therapy (ULT) in gout is known to precipitate flares, which can lead to decreased adherence and suboptimal outcomes. Until recently, there has been limited data comparing the risk of flare complicating the initiation and escalation of allopurinol and febuxostat, administered as part of a treat-to-target strategy with appropriate anti-inflammatory flare prophylaxis. The STOP Gout study (O’Dell J et al. NEJM Evid, 2022) provided an opportunity to compare flare risk with different agents and to identify predictors for gout flare during ULT initiation and titration.
Methods: The STOP Gout Study was a randomized, double-blind, placebo-controlled, 72-week study comparing allopurinol to febuxostat with a primary outcome of flare occurring during the final study phase (weeks 48-72). This post-hoc analysis evaluated flares occurring during phase 1 (weeks 0-24) when ULT was initiated and titrated towards a serum urate (sUA) goal of < 6 mg/dl (<5 mg/dl if tophi). Flares were assessed at regular intervals and based on patient report. Predictors of flare were examined using multivariable Cox proportional hazards regression with the at-risk period starting at enrollment and extending to the first of: flare, loss to follow-up or withdrawal, death, or the end of phase 1. In addition to ULT (febuxostat vs. allopurinol), baseline covariates included demographics (age, sex, race), gout-specific factors (prophylaxis used, disease duration, tophi, sUA, prior allopurinol use, CRP), and comorbidities (stage 3 chronic kidney disease [CKD], hypertension, cardiovascular disease [CVD], diabetes, diuretic use, body mass index [BMI], and alcohol use). ULT dose escalation was modeled as a time-varying covariate based on the occurrence of any dose escalation, examining for evidence of ULT-escalation interaction.
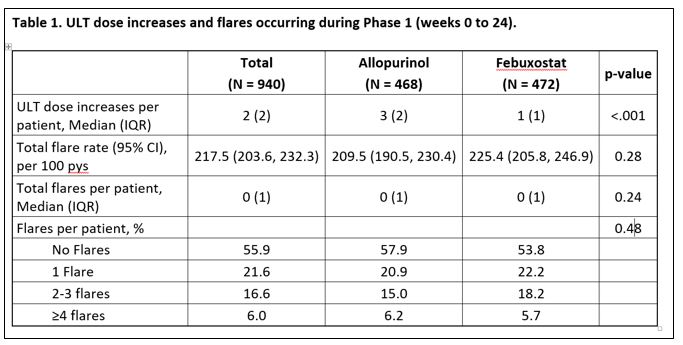
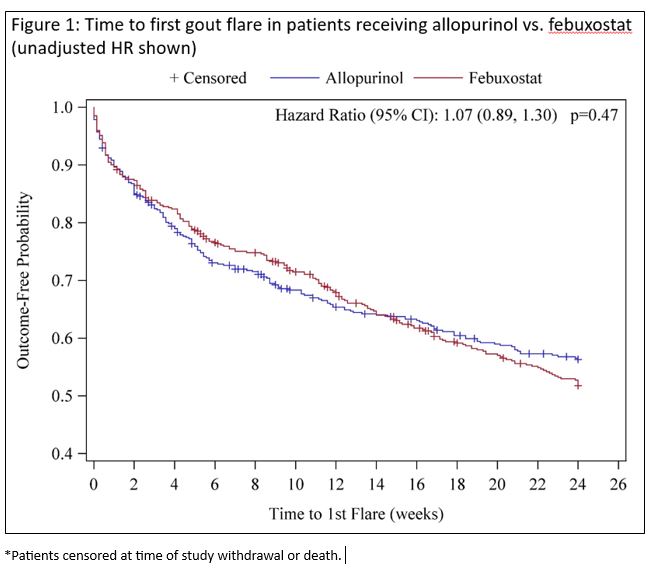
Results: Study participants (n=940) were mean age 62.1 years and predominantly male (98.4%); 468 patients received allopurinol, 472 received febuxostat. The mean sUA was 8.5 mg/dL and all participants received prophylaxis (90% colchicine). ULT dose increases and flares experienced during phase 1 are summarized in Table 1, with flare rate not differing by treatment. Time to initial flare is shown in Figure 1. In multivariable model, there was no association of ULT treatment (aHR 1.14; 95% CI 0.87-1.49), ULT escalation (aHR 1.14; 95% CI 0.90-1.44), prophylaxis type, or comorbidity with flare and no evidence of a ULT-escalation interaction (p=0.66). In the multivariable model, factors associated with increased flare risk during ULT initiation and escalation included younger age (aHR 0.99, 95% CI 0.98-1.00), higher baseline sUA (aHR 1.09; 95% CI 1.01-1.18) and absence of tophi (aHR 0.70; 95% CI 0.54-0.91).
Conclusion: In this analysis of the STOP Gout trial, we observed no difference in flare risk between allopurinol and febuxostat during initiation and titration according to a treat-to-target management strategy using best practice gout flare prophylaxis. Neither the prophylaxis employed, nor the presence of other chronic conditions that frequently accompany gout influenced flare risk following ULT initiation. Few factors were predictive of flare during ULT initiation and titration.
To cite this abstract in AMA style:
Barry A, Helget L, Androsenko M, Wu H, Kramer B, Newcomb J, Brophy M, Davis-Karim A, England B, Ferguson R, Pillinger M, Neogi T, Palevsky P, O'Dell J, Mikuls T. Gout Flares During the Initiation and Escalation of Treat-to-Target Urate Lowering Therapy: A Post-hoc Analysis of a Randomized Multicenter Comparative Effectiveness Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/gout-flares-during-the-initiation-and-escalation-of-treat-to-target-urate-lowering-therapy-a-post-hoc-analysis-of-a-randomized-multicenter-comparative-effectiveness-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gout-flares-during-the-initiation-and-escalation-of-treat-to-target-urate-lowering-therapy-a-post-hoc-analysis-of-a-randomized-multicenter-comparative-effectiveness-trial/