Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
In Germany no prospective data are available for Golimumab (GLM) in patients with RA, AS and PsA
regarding outcomes evaluating work productivity and activity in daily life.
It is the aim of this study to show the benefit of GLM in work productivity and activity for RA, AS and PsA pts in Germany. The analysis was performed by the validated WPAI (Work Productivity Activity Impairment) as primary endpoint. WPAI is rated to be the most psychometrically validated and frequently used instrument for measuring of health-related work-productivity. Methods:
As primary endpoint the change of work productivity impairment and ability for daily activities in month 3 (V1) versus baseline visit (V0) was evaluated.
All 4 subscores of the WPAI were analyzed: disease related absence from work (absenteeism), working while sick (presenteeism), total work productivity impairment (TWPI) and activity impairment with TWPI as primary score.
In addition an evaluation of the activity impairment in the mITT population (modified-Intention-To-Treat)
was performed. Results:
749 pts were included in the study (all patients who started with GLM); thereof 664 pts formed the mITT population (at least V0 and V1 documented). 469 (RA=150, AS=168, PsA=151) of 664 pts were included in the analysis of the primary efficacy endpoint, as they were employed at V0.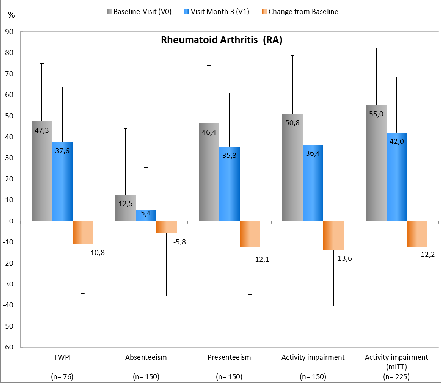 Figure 1: Overview of WPAI scores including relevant standard deviations of RA pts; for all p<0.05 (mean difference of TWPI is based on 76 / 150 pts, due to the fact that for 74 pts entries for the WPAI questionnaire were missing).
Figure 1: Overview of WPAI scores including relevant standard deviations of RA pts; for all p<0.05 (mean difference of TWPI is based on 76 / 150 pts, due to the fact that for 74 pts entries for the WPAI questionnaire were missing).
 Figure 2: Overview of WPAI scores including relevant standard deviations of AS pts; for all p<0.05 (mean difference of TWPI is based on 104 / 168 pts, due to the fact that for 64 pts entries for the WPAI questionnaire were missing).
Figure 2: Overview of WPAI scores including relevant standard deviations of AS pts; for all p<0.05 (mean difference of TWPI is based on 104 / 168 pts, due to the fact that for 64 pts entries for the WPAI questionnaire were missing).
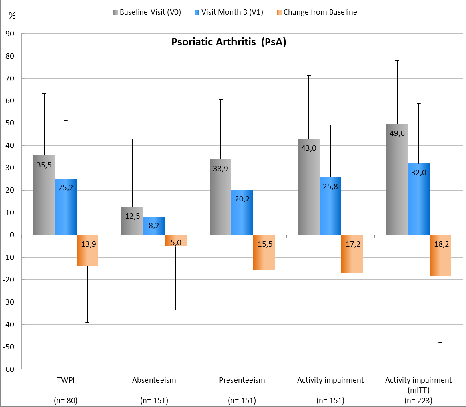 Figure 3: Overview of WPAI scores including relevant standard deviations of PsA pts; for all p<0.05 (mean difference of TWPI is based on 80 / 151 pts, due to the fact that for 71 pts entries for the WPAI questionnaire were missing). Conclusion:
Figure 3: Overview of WPAI scores including relevant standard deviations of PsA pts; for all p<0.05 (mean difference of TWPI is based on 80 / 151 pts, due to the fact that for 71 pts entries for the WPAI questionnaire were missing). Conclusion:
GLM s.c. 1 x monthly is an effective treatment in pts. with RA, AS and PsA.
All scores of the WPAI showed a significant (p< 0.05) reduction in mean score values in each indication. GLM leads to an improvement of work productivity and daily activities in all pts. already within the first 3 months of treatment.
To cite this abstract in AMA style:
Krüger K, Remstedt S, Thiele A, Klaudius I. Golimumab Improves Work Productivity and Activity Impairment in Patients with Rheumatoid Arthritis (RA), Ankylosing Spondylitis (AS) and Psoriatic Arthritis (PsA): Interim Results from a Non-Interventional Trial in Germany [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/golimumab-improves-work-productivity-and-activity-impairment-in-patients-with-rheumatoid-arthritis-ra-ankylosing-spondylitis-as-and-psoriatic-arthritis-psa-interim-results-from-a-non-intervent/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/golimumab-improves-work-productivity-and-activity-impairment-in-patients-with-rheumatoid-arthritis-ra-ankylosing-spondylitis-as-and-psoriatic-arthritis-psa-interim-results-from-a-non-intervent/
