Session Information
Date: Sunday, October 26, 2025
Title: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti–synthetase syndrome is generally responsive to glucocorticoid (GC) therapy, though relapse frequently complicates tapering. Prolonged GC use is associated with adverse events and decreased quality of life, yet the optimal maintenance dose to prevent relapse remains unclear. While immunosuppressants (IS) are often used in combination with GC to prevent disease recurrence, the risk of flare in patients treated with both remains insufficiently understood.
Methods: We retrospectively reviewed patients with anti–aminoacyl–tRNA synthetase (anti–ARS) antibody-positive myositis, identified by enzyme immunoassay (LSI Medience), treated at our institution between April 2018 and September 2024. Among 45 patients identified, 37 who received GC and had ≥26 weeks of follow-up were included. Clinical characteristics, relapse, GC tapering, and IS use were analyzed. Multivariate Cox proportional hazards, ROC curve, and Kaplan–Meier analyses were performed.
Results: Of the 37 patients, 21 were female (57%) with a mean age of 64 years; 33 had interstitial lung disease. Methylprednisolone pulse therapy was given in 10 cases; the average initial prednisolone (PSL) dose was 39 mg/day (0.7 mg/kg). IS was used in 36 of 37 cases (97%). Anti–Jo–1 antibodies were positive in 9 patients, non–Jo–1 in 26, and untyped in 2. Relapse occurred in 5 Jo–1-positive (56%) and 16 non–Jo–1 (62%) patients (p = 1.00). No relapses were observed in the Jo–1 group when PSL reached 5 mg/day, while 5 relapses occurred in the non–Jo–1 group. The mean PSL dose at relapse was significantly lower in the Jo–1 group (1.0 vs. 4.3 mg/day; p = 0.039).In multivariate Cox analysis, Raynaud’s phenomenon (HR 3.8; 95% CI 1.18–12.3; p = 0.026) and longer interval until IS initiation (HR 1.01; 95% CI 1.00–1.03; p = 0.018) were independently associated with relapse. ROC analysis identified 1.55 mg/day as the optimal PSL cutoff to predict non-relapse (sensitivity 66.7%, specificity 68.8%). Relapse was more common in patients tapered to < 5 mg/day than those on ≥5 mg/day. KM analysis from week 26 to 104 showed a trend toward more relapse in patients < 3 mg/day (log-rank p = 0.196).
Conclusion: In this IS-treated cohort, Jo–1-positive patients were able to maintain remission on lower GC doses, with no relapses observed when PSL was tapered to 5 mg/day. However, relapse was more frequent in patients tapered below 5 mg/day, and a possible trend toward increased relapse was also observed below 3 mg/day. Although nearly all patients received IS, delayed initiation was associated with increased relapse risk. These findings support early immunosuppressant use and strategic tapering guided by clinical risk factors such as the presence of Raynaud’s phenomenon and timing of IS initiation. Tapering GC doses to 3 mg/day may be possible with caution, although relapse risk should be carefully monitored in anti–synthetase syndrome.
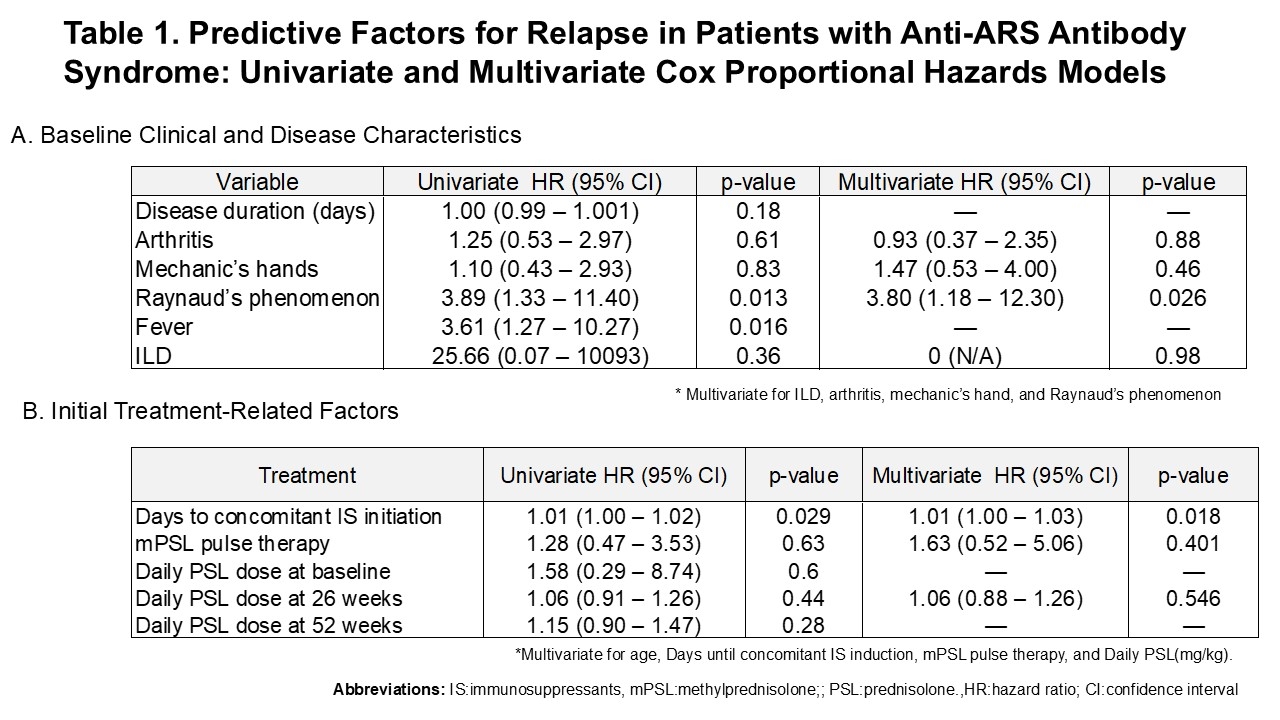 Table 1. Predictive Factors for Relapse in Patients with Anti-ARS Antibody Syndrome: Univariate and Multivariate Cox Proportional Hazards Models
Table 1. Predictive Factors for Relapse in Patients with Anti-ARS Antibody Syndrome: Univariate and Multivariate Cox Proportional Hazards Models
.jpg) Figure 1. Prednisolone Dose at Relapse in Anti-Jo-1 and Non-Jo-1 Anti-ARS Antibody Groups
Figure 1. Prednisolone Dose at Relapse in Anti-Jo-1 and Non-Jo-1 Anti-ARS Antibody Groups
.jpg) Figure2:Kaplan–Meier Curve for Relapse-Free Survival According to Prednisolone Dose at Week 26 ( < 3 mg/day vs ≥3 mg/day)
Figure2:Kaplan–Meier Curve for Relapse-Free Survival According to Prednisolone Dose at Week 26 ( < 3 mg/day vs ≥3 mg/day)
To cite this abstract in AMA style:
Inoue A, Yamane T, Yasuda N, Ohnishi T. Glucocorticoid Tapering and Relapse Risk in Anti-ARS Antibody-Positive Myositis: A Retrospective Study in a Single-Center Cohort Treated with Immunosuppressants [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/glucocorticoid-tapering-and-relapse-risk-in-anti-ars-antibody-positive-myositis-a-retrospective-study-in-a-single-center-cohort-treated-with-immunosuppressants/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoid-tapering-and-relapse-risk-in-anti-ars-antibody-positive-myositis-a-retrospective-study-in-a-single-center-cohort-treated-with-immunosuppressants/
