Session Information
Session Type: Abstract Session
Session Time: 11:30AM-11:45AM
Background/Purpose: Obesity is highly prevalent in psoriatic arthritis (PsA) and is associated with reduced therapeutic response, worse disease outcomes, and increased cardiometabolic risk. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are being increasingly used for weight loss and diabetes, but their impact on PsA outcomes remains unclear. We aimed to characterize patients with PsA initiating GLP-1 RAs and assess longitudinal changes in weight, psoriatic disease activity and cardiometabolic parameters.
Methods: We conducted a retrospective analysis of PsA patients from two academic cohorts (Toronto and NYU, PPACMAN sites) who initiated GLP-1 RAs (semaglutide, liraglutide, or tirzepatide) for weight loss or diabetes. Data from clinic visits within 1 year before and after GLP-1 RA initiation were extracted, including joint counts, skin involvement, enthesitis, CRP, and patient-reported outcomes. Disease Activity in PsA (DAPSA) scores were calculated. Cardiometabolic data (weight, blood pressure, lipids) were also collected. Within-patient changes were assessed using the Wilcoxon signed-rank test. Linear regression models, adjusting for baseline outcome values, were used to examine the association between percent weight change and change in outcomes.
Results: A total of 48 patients (18 Toronto, 30 NYU; median age 52.7 years; 60.4% female; median BMI 34.9, Table 1) were included. Cardiometabolic and other comorbidities were common: obesity (64.6%), diabetes (35.4%), hypertension (50%), cardiovascular disease (12.5%), fatty liver (20.8%), depression (39.6%), sleep apnea (35.4%), and osteoarthritis (27.1%). Sixty percent had ≥3 comorbidities. Significant weight loss was observed post-treatment (-6.43 kg (95% CI -9.5, -2.0), p< 0.0001, Table 2), with 35.4% losing 5–10% and 25% losing >10% of their baseline weight. CRP levels decreased significantly (-1.1 mg/L (95% CI: -2.0, -0.2), p=0.002), as did pain scores (-1.0 (95% CI: -1.75, 0), p=0.01). DAPSA (-3.52, p=0.11) and patient global assessment (-0.5, p=0.08) showed numerical improvements. A significant reduction in triglycerides (-0.35 mmol/L, p=0.02) was also noted. Regression analyses revealed that each 1% reduction in body weight was associated with significant improvements in DAPSA (β=-0.49 (95% CI: -0.94, -0.03), Table 3), tender joint count (β=-0.18 (95% CI: -0.32, -0.05)), EQ-5D (β=0.0016 (95% CI: 0.008, 0.023)), LDL (β=-0.05 (95% CI: -0.10, -0.003)), and systolic blood pressure (β=-0.67 (95% CI: -1.18, -0.15)).
Conclusion: In this initial analysis of a real-world study conducted in dual psoriasis-PsA PPACMAN clinics, GLP-1 RA therapy in patients with PsA was associated with clinically meaningful weight loss and improvements in systemic inflammation, pain, and cardiometabolic markers. Improvements in PsA outcomes were proportional to the degree of weight loss. These findings warrant further investigation in prospective controlled studies to evaluate the therapeutic role of GLP-1 RAs in PsA management and its frequent comorbidities.
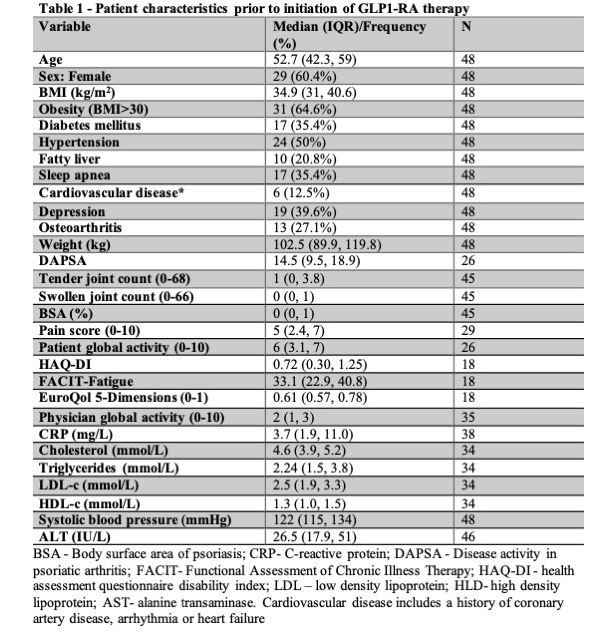 Table 1 – Patient characteristics prior to initiation of GLP1-RA therapy
Table 1 – Patient characteristics prior to initiation of GLP1-RA therapy
.jpg) Table 2 – Median changes in PsA and metabolic measure following GLP1-RA therapy
Table 2 – Median changes in PsA and metabolic measure following GLP1-RA therapy
.jpg) Table 3 – The association between body weight reduction (% from baseline) and changes in PsA and metabolic outcomes following GLP1-RA therapy – Linear regression model
Table 3 – The association between body weight reduction (% from baseline) and changes in PsA and metabolic outcomes following GLP1-RA therapy – Linear regression model
To cite this abstract in AMA style:
Eder L, Scher U, Chen K, Rice A, Thib S, Haberman R. Glucagon-like peptide-1 receptor agonists therapy is associated in improvement in psoriatic arthritis-related and metabolic outcomes: A retrospective analysis of two cohorts [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/glucagon-like-peptide-1-receptor-agonists-therapy-is-associated-in-improvement-in-psoriatic-arthritis-related-and-metabolic-outcomes-a-retrospective-analysis-of-two-cohorts/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucagon-like-peptide-1-receptor-agonists-therapy-is-associated-in-improvement-in-psoriatic-arthritis-related-and-metabolic-outcomes-a-retrospective-analysis-of-two-cohorts/
