Session Information
Date: Saturday, November 16, 2024
Title: Plenary I
Session Type: Plenary Session
Session Time: 9:15AM-10:45AM
Background/Purpose: Therapeutic advances of the recent past have led to significant changes in clinical practice when treating patients with psoriatic arthritis (PsA). Since the advent of biological disease modifying anti-rheumatic drugs (DMARD), double-blind randomized controlled trials (RCT) have been the mainstay of drug development. In rheumatoid arthritis clinical trials, over time, a significant increase in placebo response (PBO) rates has been observed, and it is unclear whether this likewise applies to PsA clinical trials. We also hypothesized that one element contributing to increasing placebo responses are changing recruitment patterns with an increasing move towards geographic areas with less affluent health care systems.
Methods: We analysed the placebo arms of all available trials with reports on ACR response rates at week 12. To ensure completeness of trial acquisition, we identified all placebo controlled RCTs investigating biological or targeted synthetic DMARDs in PsA patients with established csDMARD background therapy until December 19th, 2023. First, we quantified the association of ACR20 PBO rates with the study start year. Next, to assess geographic distribution, we extracted the numbers of recruiting centres per country from the original articles or from clinicaltrials.gov. We then used world bank data on the per capita gross national income (GNI) of each recruiting country to calculate a weighted GNI per study and performed linear mixed model regression with a random effect on study level, weighted by the number of patients per study, and adjusted for the global trend of rising GNPs over time. Statistics were conducted using R (version 4.3.1).
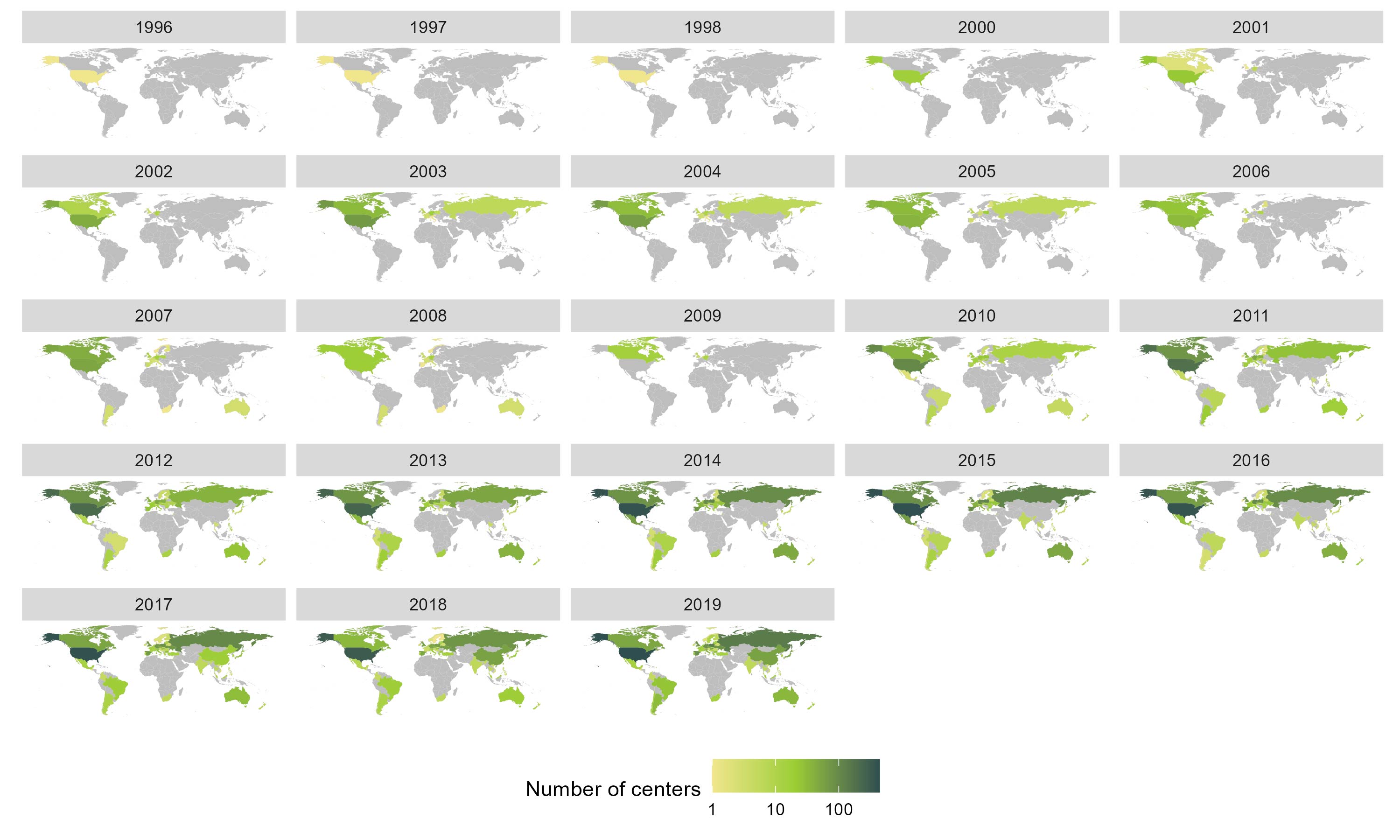
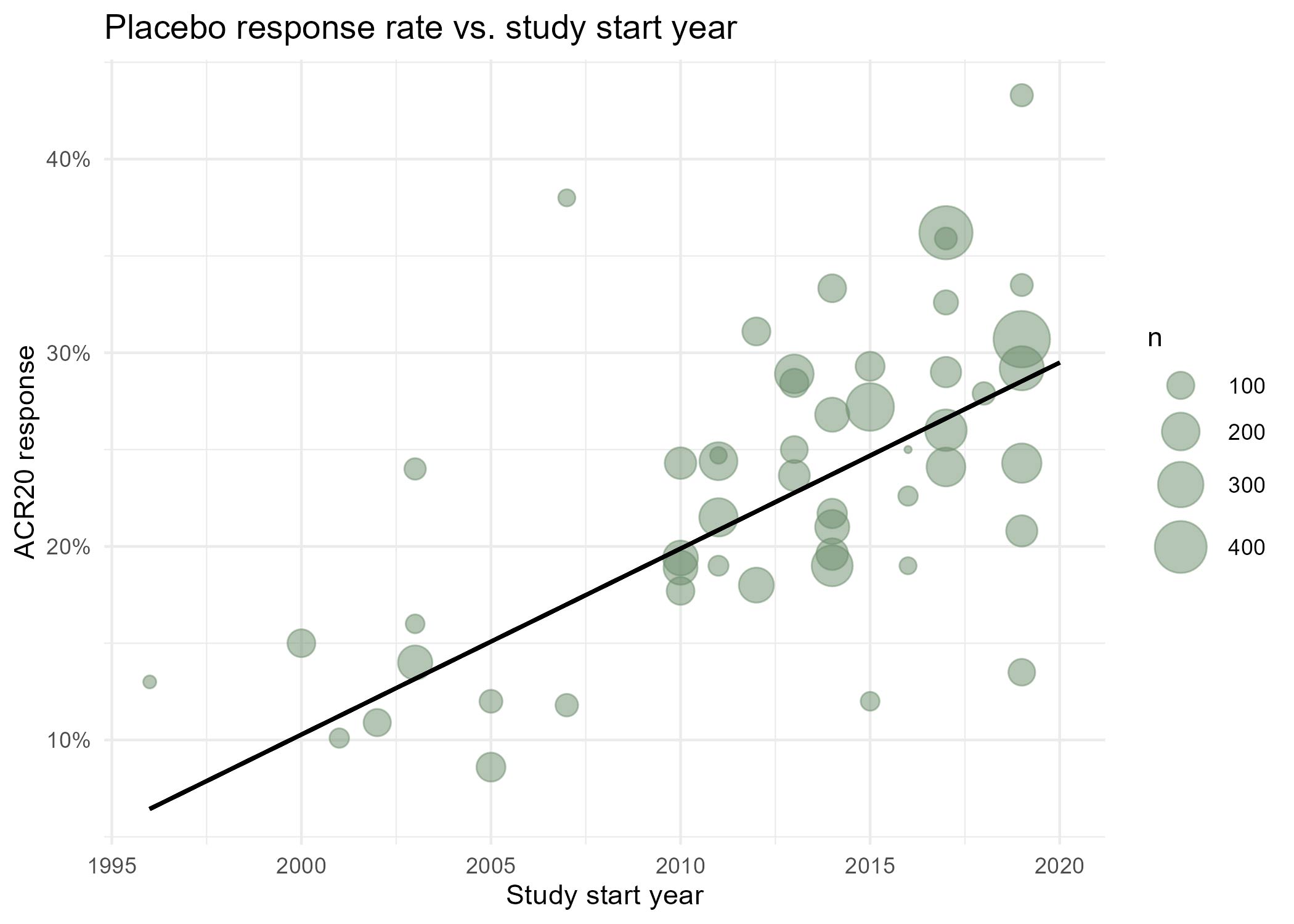
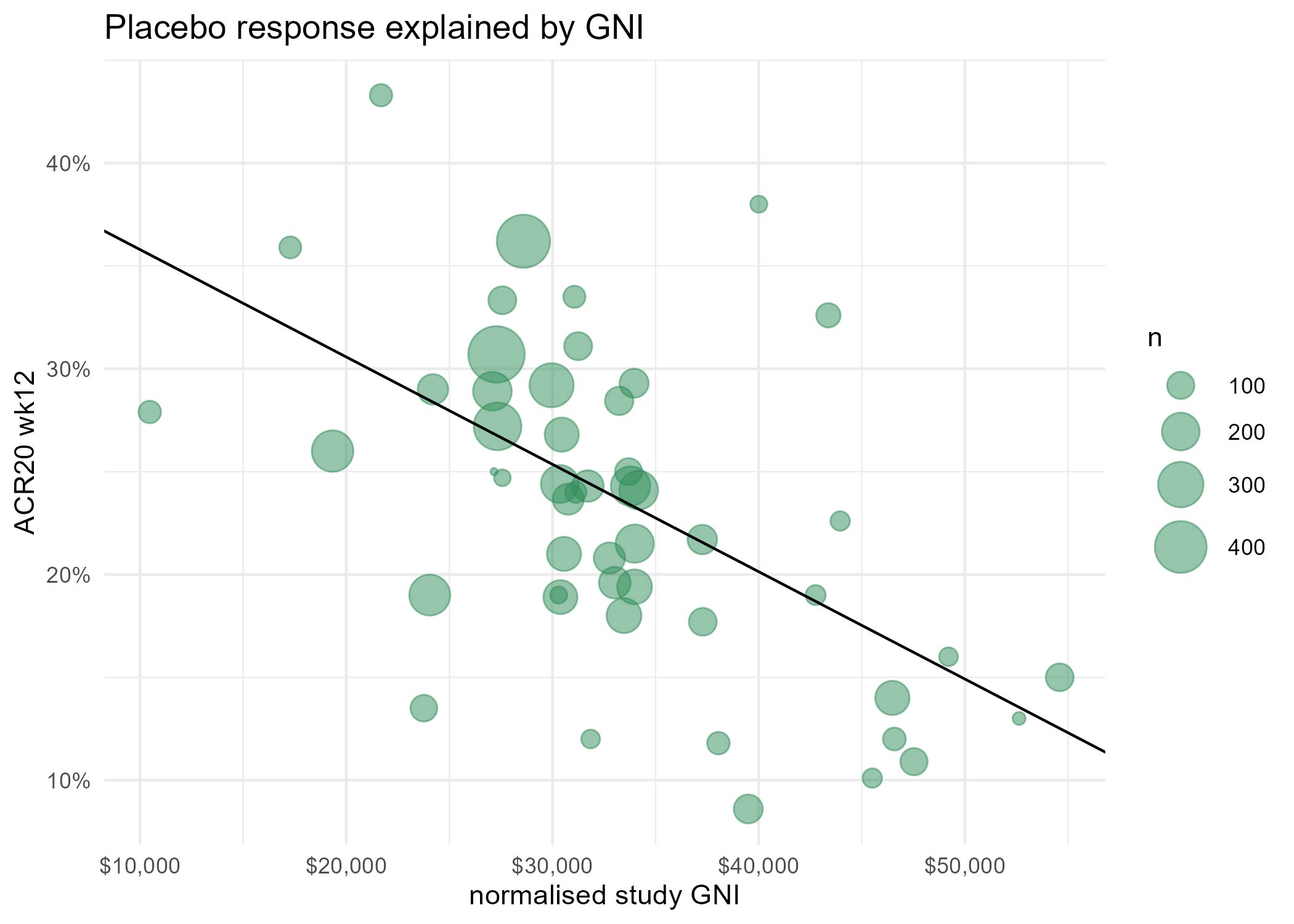
Results: For complete trial evaluation, two independent researchers screened in total 2.389 articles, of which 154 were assessed in detail, with 51 reports finally fulfilling the criteria for trial analysis. Of the included studies the year of study start ranged from 1996 to 2019. A significant positive association of PBO with the study start year was found (estimate: 0.92±0.16;p< 0.001). Recruitment areas have expanded from initially covering only North America to an increasing global recruitment over time (Figure 1), with more recent studies being conducted in less affluent countries. We identified a strong and significant negative association of the weighted and normalised GNP per study and PBO rates in a study, with an estimate of an ACR20 response -5.22%±1.13 per 10.000 international Dollars (p< 0.001;Figure 2).
Conclusion: PBO rates are rising over time, in parallel to the change of geographic recruiting patterns, with more recent RCTs recruiting increasingly in less affluent countries. Our data support the hypothesis that geographic recruitment changes are a significant contributor to the higher placebo rates observed in more recent clinical trials. One explanation may be the more limited access to health care and innovative therapies in less affluent countries, leading to preference for inclusion in clinical trials and subsequently also higher regression to the mean, which manifests as placebo response. These effects should have implications for sponsors, investigators, patients, health care providers and political stakeholders.
To cite this abstract in AMA style:
Kerschbaumer A, Steiner M, Khalili S, Aletaha D, Smolen J. Global Recruiting Patterns Are Associated with Placebo Response Rates in Clinical Trials of Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/global-recruiting-patterns-are-associated-with-placebo-response-rates-in-clinical-trials-of-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/global-recruiting-patterns-are-associated-with-placebo-response-rates-in-clinical-trials-of-psoriatic-arthritis/