Session Information
Date: Monday, October 27, 2025
Title: (0955–0977) Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
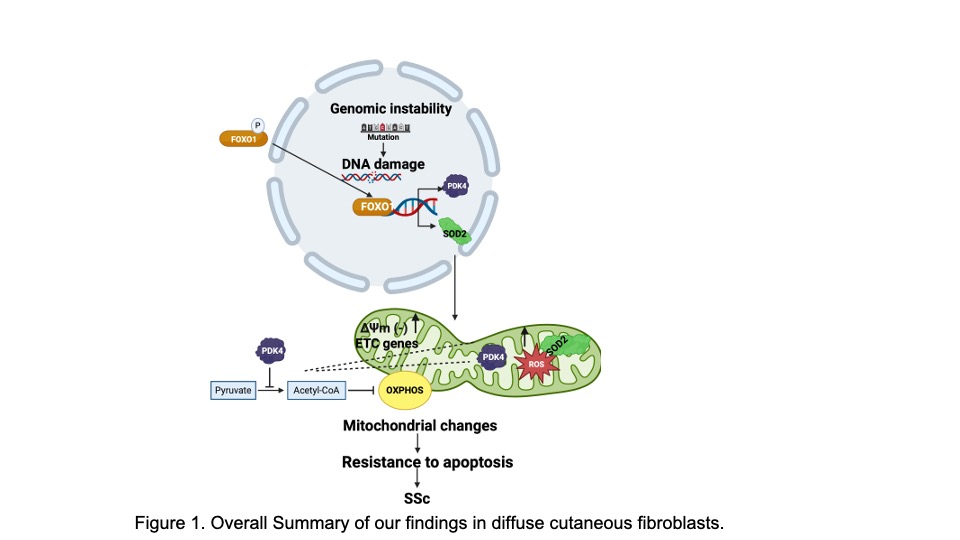
Background/Purpose: Systemic sclerosis (SSc) is a life-threatening autoimmune disease with limited treatment options, including autologous hematopoietic stem cell transplantation (AHSCT). We recently showed that dermal fibroblasts (DFs) in patients with diffuse cutaneous SSc (dcSSc) develop a cancer-like phenotype characterized by genomic instability and increased double-stranded DNA breaks (DSBs). However, little is known about the mechanisms promoting DF survival following the accumulation of genomic mutations and the effects of AHSCT on mutational frequency. We hypothesized that in response to reactive oxygen species (ROS), dcSSc DFs may promote a mitochondrial-dependent, resistance-to-apoptosis via the activation of the transcription factor forkhead box 1(FOXO1).
Methods: We characterized mutational frequencies and signatures in dcSSc (N =35) and AHSCT patients (Nf9), using whole exome sequencing (WES) of dermal sections. We generated DFs from dcSSc, post-AHSCT (or age/sex matched health subjects). We quantified the frequency of DSBs by measuring γ-H2AX levels (immunoblot (IB)), and DSB nuclear foci (confocal microscopy). ROS and mitochondrial membrane potential were measured in DFs using flow cytometry. Changes in mitochondrial dynamics (fusion/fission, via IB), biogenesis (qRT-PCR), and morphology (confocal microscopy) were assessed. FOXO1 activation was determined by measuring nuclear (active) FOXO1, and expression of its downstream mRNA targets SOD2 and PDK4. Resistance-to-apoptosis was determined at baseline, following treatment with cyclophosphamide, a FOXO1-inhibitor, or with overexpression of constitutively active FOXO1 using TUNEL and cleaved caspase 3 levels (IB).
Results: dcSSc patients had a reduced mutational frequency in both the dermis and in DFs compared to patients treated with AHSCT. We detected a base-excision repair signature (which is promoted by increased ROS levels) in dcSSc DFs. This was associated with an increased frequency of DSBs and ROS in dcSSc DF compared to post-AHSCT and HC. dcSSc DF had increased indicators associated with mitochondrial remodelling, mitochondrial biogenesis, and mitochondrial fusion. Importantly, FOXO1 was exclusively activated in dcSSc, but not HC or post-AHSCT DF, and its activation was required for promoting apoptosis resistance. Finally, overexpression of constitutively active FOXO1 in HC DF resulted in similar metabolic changes as seen in dcSSc and increased DF survival.
Conclusion: Our study highlights a novel mechanism whereby genotoxic stress signals in dcSSc promote cell survival via metabolic stress remodeling (Fig.1). It also provides mechanistic insights for the effects of AHSCT on the mutational landscape in dcSSc. Future studies targeting this pathway may implicate this novel mitochondrial/FOXO1 pathway as a novel therapeutic strategy in patients with dcSSc.
To cite this abstract in AMA style:
Khan L, Wang J, Iyer A, Redmond D, Hennessey D, O'Keefe S, Storek J, van Eeden C, Gniadecki R, Osman M. Genomic instability in systemic sclerosis is promoted by metabolic remodelling via a FOXO1-dependent axis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/genomic-instability-in-systemic-sclerosis-is-promoted-by-metabolic-remodelling-via-a-foxo1-dependent-axis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/genomic-instability-in-systemic-sclerosis-is-promoted-by-metabolic-remodelling-via-a-foxo1-dependent-axis/