Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Systemic Sclerosis (SSc) is an autoimmune disease characterized by diffuse fibrosis and vasculopathy that has potential to affect nearly every organ system. Interstitial lung disease (ILD) is present in up to 90% of SSc patients and the leading cause of SSc-related mortality. Difficulty in managing SSc-ILD stems from the limited understanding of the molecular mechanisms underpinning the disease. Previous work from our lab suggests a subset of SSc-ILD patients are characterized by shortened telomeres and increased expression of markers of cellular senescence (p16 and p21). Here we present a comprehensive gene expression meta-analysis in SSc-ILD lung tissues and healthy controls to identify pathologic genes and pathways, while specifically probing aging and cellular senescence pathways.
Methods: Three publicly available gene expression microarray data sets of the lung including 42 SSc-ILD subjects and 18 healthy controls were identified from the Gene Expression Omnibus (GEO). A meta-analysis was performed by first processing each data set individually according to their unique platform then cross platform normalization and data set merging to create a gene expression matrix using ComBat. Single-gene and network analyses using LIMMA, ClusterProfiler and GSEA were conducted to identify genes and pathways significantly associated with SSc-ILD. Enrichment of previously established aging and cell specific senescence signatures was assessed using hypergeometric testing.
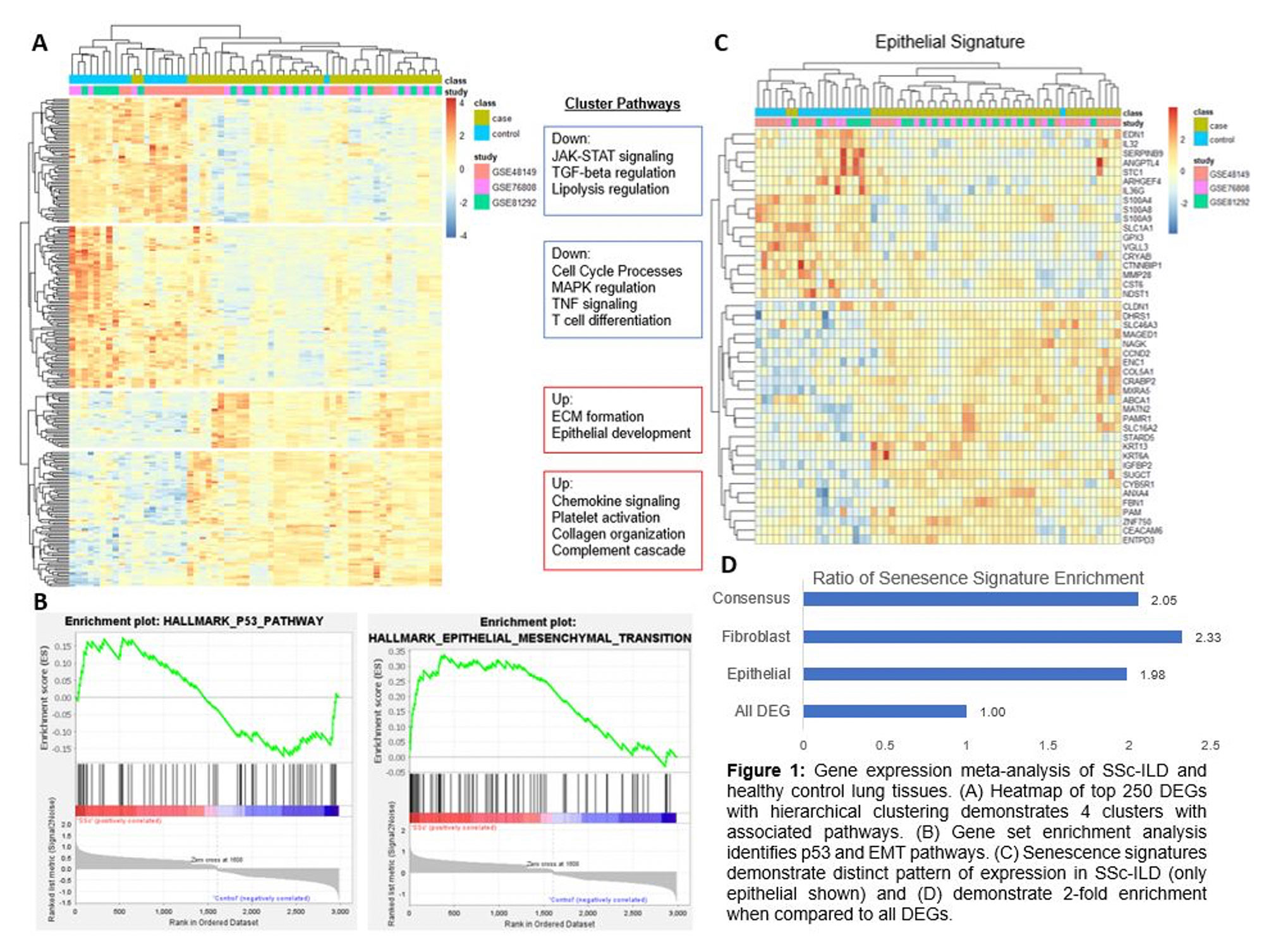
Results: There were 586 differentially expressed genes (DEGs) (FC >1.3, q< 0.05) and 89 relevant profibrotic and non-fibrotic pathways in SSc-ILD (q< 0.05). Among the top upregulated DEGs, notable markers included MMP7, a metalloproteinase involved in extracellular matrix (ECM) degradation and biomarker of pulmonary fibrosis, KRT17, an epithelial basal cell marker overexpressed in idiopathic pulmonary fibrosis, and GDF15, a marker of cellular stress and senescence. Using hierarchical clustering based on the top 250 DEGs, 4 distinct clusters of genes emerged with pathway analysis of individual clusters demonstrating notable down regulation of cell processes and up regulation of ECM formation and collagen organization (Fig 1a). In gene set enrichment analysis, p53 and epithelial mesenchymal transition (EMT) pathways were the most highly enriched in SSc-ILD (Fig 1b). The p53 signaling pathway is hypothesized to promote lung fibrosis through apoptosis, DNA damage, and oxidative stress of lung epithelia and is also upregulated in IPF. When probing senescence signatures, we found a clear separation based on hierarchical clustering between SSc-ILD and controls (Fig 1c) and an average 2-fold enrichment of these signatures in SSc-ILD compared to all other DEGs (p< 0.05) (Fig 1d).
Conclusion: While there is a diversity of DEGs in SSc-ILD, there is a striking enrichment of aging and cellular senescence related pathways including p53 signaling pathways and epithelial and fibroblast senescence signatures. These findings demonstrate the prominent presence of cellular senescence in SSc-ILD and suggest it may be a mechanistic driver of disease pathogenesis.
To cite this abstract in AMA style:
Yang M, Lee S, Neely J, Sirota M, Wolters P. Gene Expression Meta-Analysis Reveals Aging and Cellular Senescence Signatures in Scleroderma-associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/gene-expression-meta-analysis-reveals-aging-and-cellular-senescence-signatures-in-scleroderma-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gene-expression-meta-analysis-reveals-aging-and-cellular-senescence-signatures-in-scleroderma-associated-interstitial-lung-disease/