Session Information
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease with a strong genetic association to Class II HLA-DRB1*04:01. Presentation of arthritogenic peptides bound to DRB1*04:01 results in activation of autoreactive T cells which then mediate arthritic inflammation. Our previous work has demonstrated that a single amino acid change from lysine (K) to glutamic acid (E) at position 71 of DRB1*04:01 blocks binding of collagen258-272 and abrogates collagen-specific CD4+ T cell activation without inducing alloreactivity (1). However, the K71E edit does not block binding of native or citrullinated vimentin66-78 and α-enolase11-25 peptides. Position 71 is located in pocket 4 of the peptide binding groove. Here we investigate if changing the amino acid in pocket 1 (position 86) or in pocket 2 (position 82) would eliminate binding of citrullinated arthritogenic peptides in addition to the immunodominant collagen peptide.
Methods: We edited DRB1*04:01 at position 82, from N to either leucine (L) or methionine (M) and at position 86 from glycine (G) to L. The edited DRB1*04:01N82L (N82L), DRB1*04:01N82M (N82M) and DRB1*04:01G86L (G86L) molecules were expressed in T2 cells. Peptide binding of biotinylated citrullinated and native α-enolase11-25, citrullinated and native vimentin66-78 and native collagen258-272 peptides was detected by streptavidin-PE using flow cytometry.
Results: All three edits blocked binding of the native forms of -enolase, vimentin and collagen. Citrullinated vimentin66-78 binding to DRB1*04:01N82L was reduced 85% and binding to DRB1*04:01N82M was reduced 95%. Similarly, binding of citrullinated α-enolase11-25 to DRB1*04:01N82L and to DRB1*04:01N82M was reduced by 97%. The G86L edit to DRB1*04:01 abrogated binding of citrullinated α-enolase11-25 and collagen258-272 but citrullinated vimentin66-78 could still bind.
Conclusion: Replacing N at position 82 of DRB1*04:01, with either L or M, abrogates peptide binding of citrullinated a-enolase, citrullinated vimentin and collagen. Blocking the binding of citrullinated peptides to edited DRB1*04:01 on APCs would prevent presentation of these autoantigens to autoreactive T cells. Our results demonstrate that introducing mutations at position 82 in DRB1*04:01 blocks binding of all arthritogenic peptides and thus has potential to be used as gene therapy to safely halt progression of RA in patients carrying DRB1*04:01
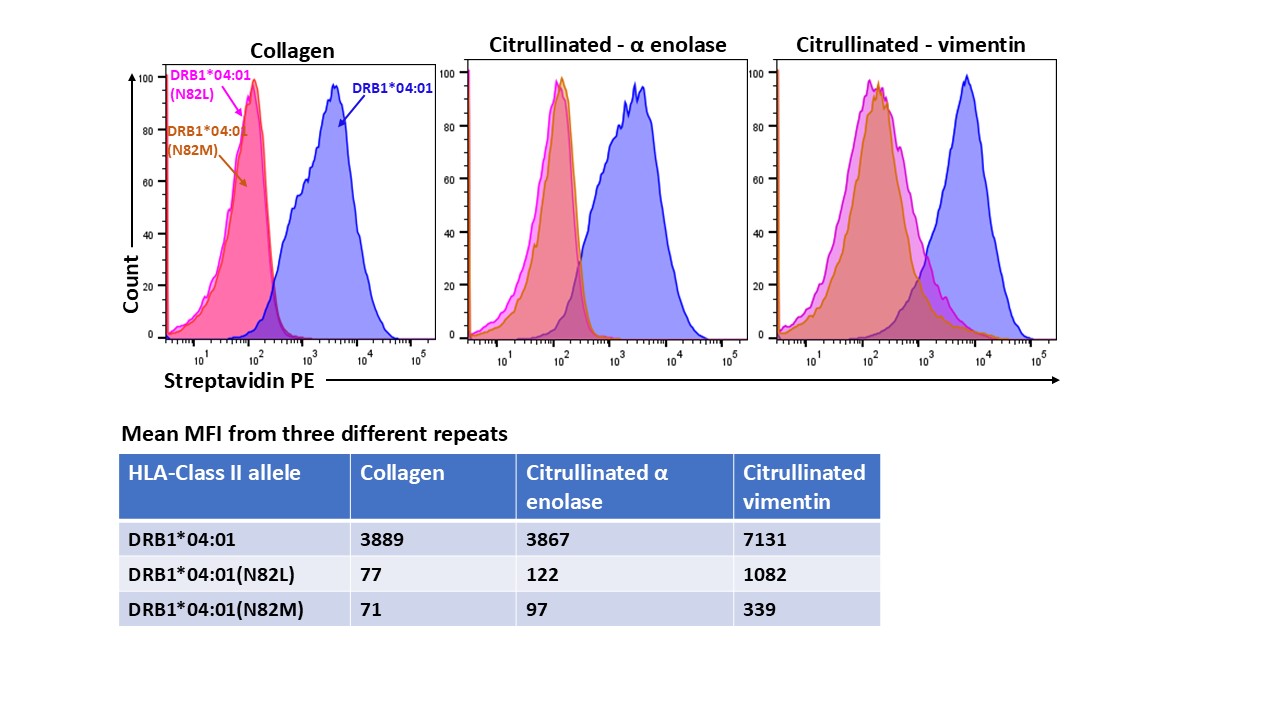 Figure 1: Binding of arthritogenic peptides with unedited DRB1*04:01, and edits at position 82
Figure 1: Binding of arthritogenic peptides with unedited DRB1*04:01, and edits at position 82
To cite this abstract in AMA style:
Jha V, Freed B, Jin N, Miglani M, Roark C. Gene Editing of HLA-Class II DRB1*04:01 at Position 82 Abrogates Binding of Citrullinated Arthritogenic Peptides and Collagen [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/gene-editing-of-hla-class-ii-drb10401-at-position-82-abrogates-binding-of-citrullinated-arthritogenic-peptides-and-collagen/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gene-editing-of-hla-class-ii-drb10401-at-position-82-abrogates-binding-of-citrullinated-arthritogenic-peptides-and-collagen/
