Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
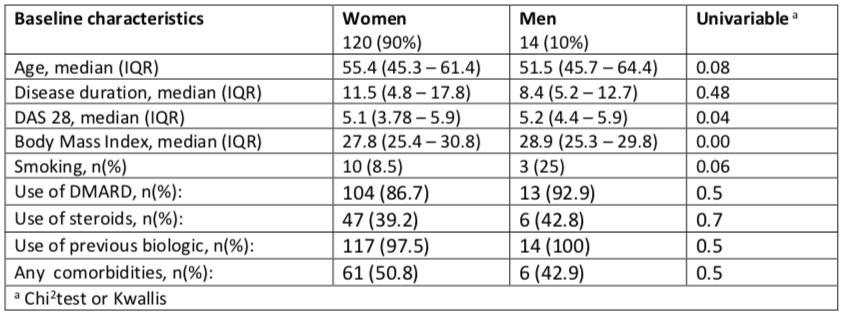
Background/Purpose: Rheumatoid arthritis (RA) is the most common autoimmune disease and is more frequent and severe in women than in men. Symptom severity, disease progression, response to therapy and overall survival differ between males and females with AR.
Determine if drug discontinuation of biologic DMARDs (bDMARDs) differs by gender in patients with rheumatoid arthritis in the Mexican Adverse Events Registry (BIOBADAMEX).
Methods: BIOBADAMEX is a Mexican ongoing cohort of patients using bDMARDs since 2016. In this analysis we included all patients with diagnosis of RA with at least two assessments. Survival on bDMARDs was estimated using Kaplan-Meier analysis. Predictors of discontinuation, including gender were investigated by Cox regression analyses.This analysis did not show a sex role on discontinuation of bDMARDs in Mexican RA patients, however we found high DAS28 had a protective role. Further longitudinal analyses will be performed including more patients to assess retention rate of bDMARDs and identify predictive variables of discontinuation.
Results: Among 727 RA patients in the cohort, 134 patients had at least two assessments, from which 121 (90%) were women. At baseline, patients had a median (IQR) disease duration of 11 (5-18) months and 55.2 (45-61) years old of age, with a median DAS28 of 5.2 (4-6). Conventional DMARDS were used by 116 (87%) patients and 53 (40%) used corticosteroids. Comorbidities were present in 66 (50%). The most common bDMARDs received at baseline were tocilizumab 31 (23%), abatacept 29(22%), adalimumab 22 (16%) and certolizumab 20 (15%). At the time of analysis, the median bDMARDs treatment duration was 17 (13-29) months, 59 (44%) had discontinued treatment, 24 for inefficacy, 17 for adverse events and 18 for other reasons. Fig 1 shows discontinuation rate curves by sex. Cox proportional-hazards demonstrated that high DAS28 >5.1 (HR 0.5, 95% CI 0.4-0.9, p=0.01) had a protective role on bDMARD discontinuation that remained in the multivariable analysis (p=0.02), while no significant differences were found regarding female sex (HR 1.4, 95% CI 0.8-2.5, p=0.26), use of corticosteroids (HR 1.3, 95% CI 0.9-1.8, p=0.20), comorbidities (HR 1.0, 95% 0.7-1.5, p=0.82) or other factors, such as age, obesity, tobacco smoking or use of conventional DMARDs.
Conclusion: This analysis did not show a sex role on discontinuation of bDMARDs in Mexican RA patients, however we found high DAS28 had a protective role. Further longitudinal analyses will be performed including more patients to assess retention rate of bDMARDs and identify predictive variables of discontinuation.
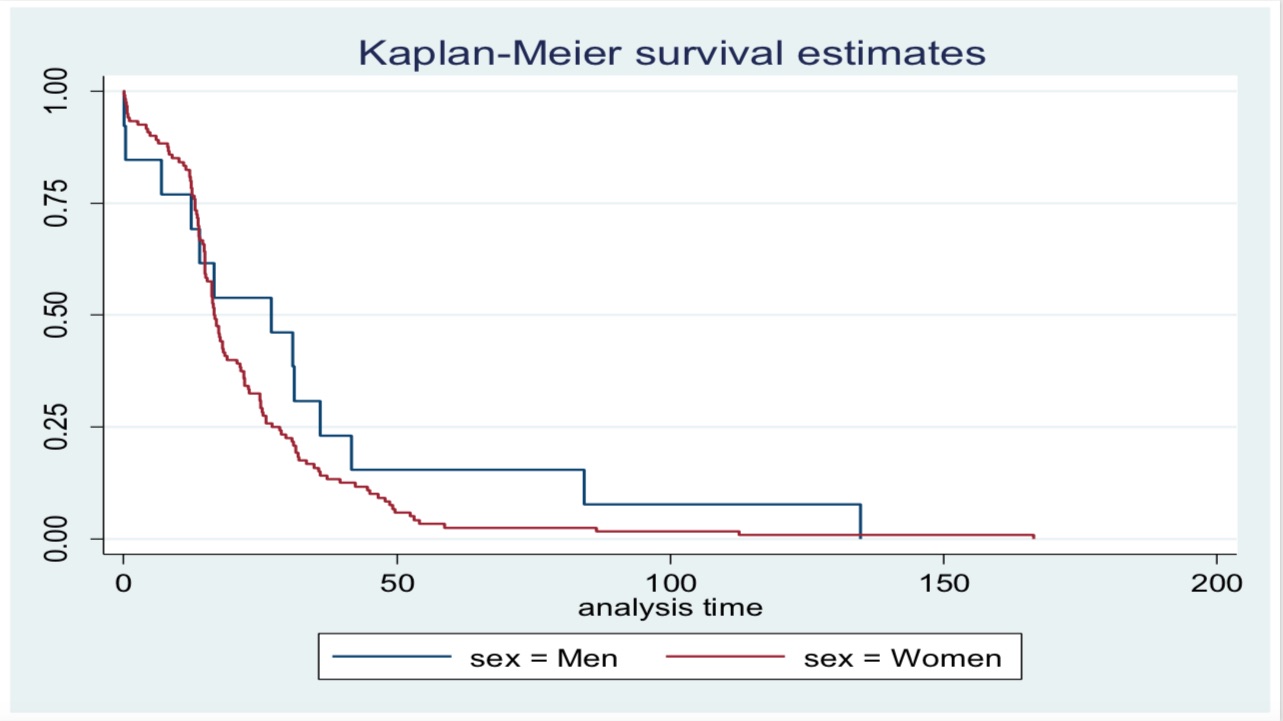 Discontinuation rate curves by sex
Discontinuation rate curves by sex
To cite this abstract in AMA style:
Rivera V, Sicsik Ayala S, Vega Morales D, Irazoque-Palazuelos F, Miranda Hernández D, Casasola Vargas J, Carrillo Vázquez S, Peña Ayala A, Castillo Ortiz A, Muñoz Monroy O, Durán Barragán S, Paz Viscarra A, Torres Valdez E, Xibille Friedmann D, Zamora Tehozol E, Valdés Corona L, Ramos Sánchez A, Santana Portillo N, Guerrero Díaz F, Vazquez Zaragoza M, Zepeda Moreno C, Alvarado Sánchez K, Rivera Valencia M, Pacheco Tena C, Alpizar-Rodriguez D. Gender and Discontinuation of Biologic DMARDs in Patients with Rheumatoid Arthritis: Data from the Mexican Biologics Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/gender-and-discontinuation-of-biologic-dmards-in-patients-with-rheumatoid-arthritis-data-from-the-mexican-biologics-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gender-and-discontinuation-of-biologic-dmards-in-patients-with-rheumatoid-arthritis-data-from-the-mexican-biologics-registry/