Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Sclerosis (SSc) is a complex, heterogeneous, multisystem autoimmune disease with high morbidity and mortality. Gastrointestinal symptoms impact more than 90% of SSc patients, ranging in severity from mild to severe and significantly impact patient function and quality of life. Prior studies have linked high intestinal permeability to severe GI involvement. This prospective study aims to elucidate the clinical correlates and impact of GI symptom severity on quality of life and assess the relationship between GI symptom severity and intestinal permeability in SSc patients.
Methods: Adult ( >18 y) SSc patients meeting the ACR/EULAR 2013 criteria with moderate-severe GI symptoms (based on UCLA-GIT 2.0) and age- and sex-matched SSc patients with none-mild GI symptoms were prospectively recruited. Patients with prior bowel surgery, new antibiotic or probiotic use in the last two weeks, active malignancy, history of inflammatory bowel disease, celiac disease, or microscopic colitis were excluded. All patients underwent a standardized clinical evaluation (including Modified Rodnan Skin Score, internal organ involvement, and Physician Global Assessment [PGA] of Disease activity) and filled a 7-day bowel diary (frequency and stool consistency). Patient-reported outcomes (SHAQ, PROMIS-29, and Patient Global Assessment [PtGA] of Disease activity) were assessed. SCTC Damage Index, Medsger Disease Severity Score, and HADS Depression and Anxiety scores were also assessed. Intestinal permeability was evaluated using a standardized 24h saccharide-based excretion assay (100 mg 13C-mannitol and 1g lactulose) in a small subset of patients (n=12-13/group).
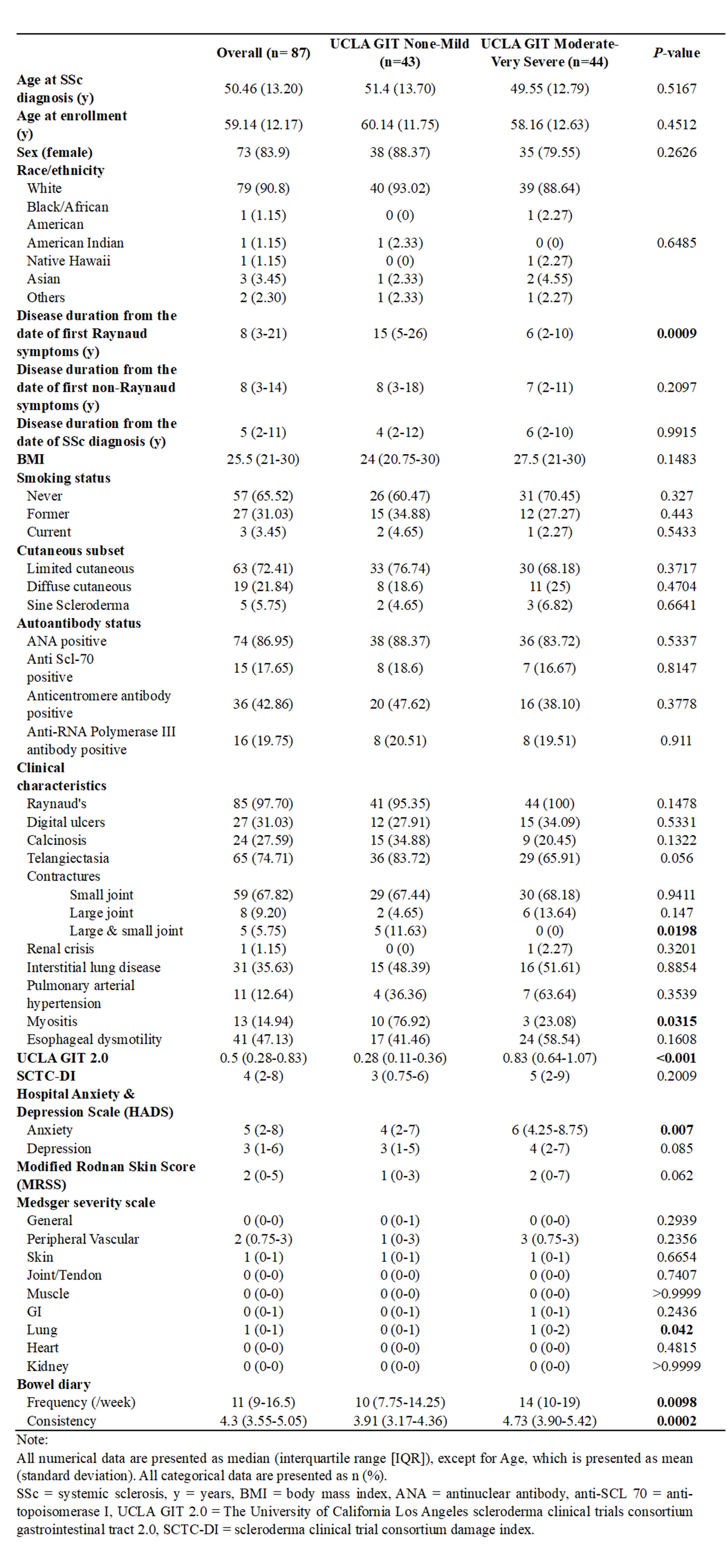
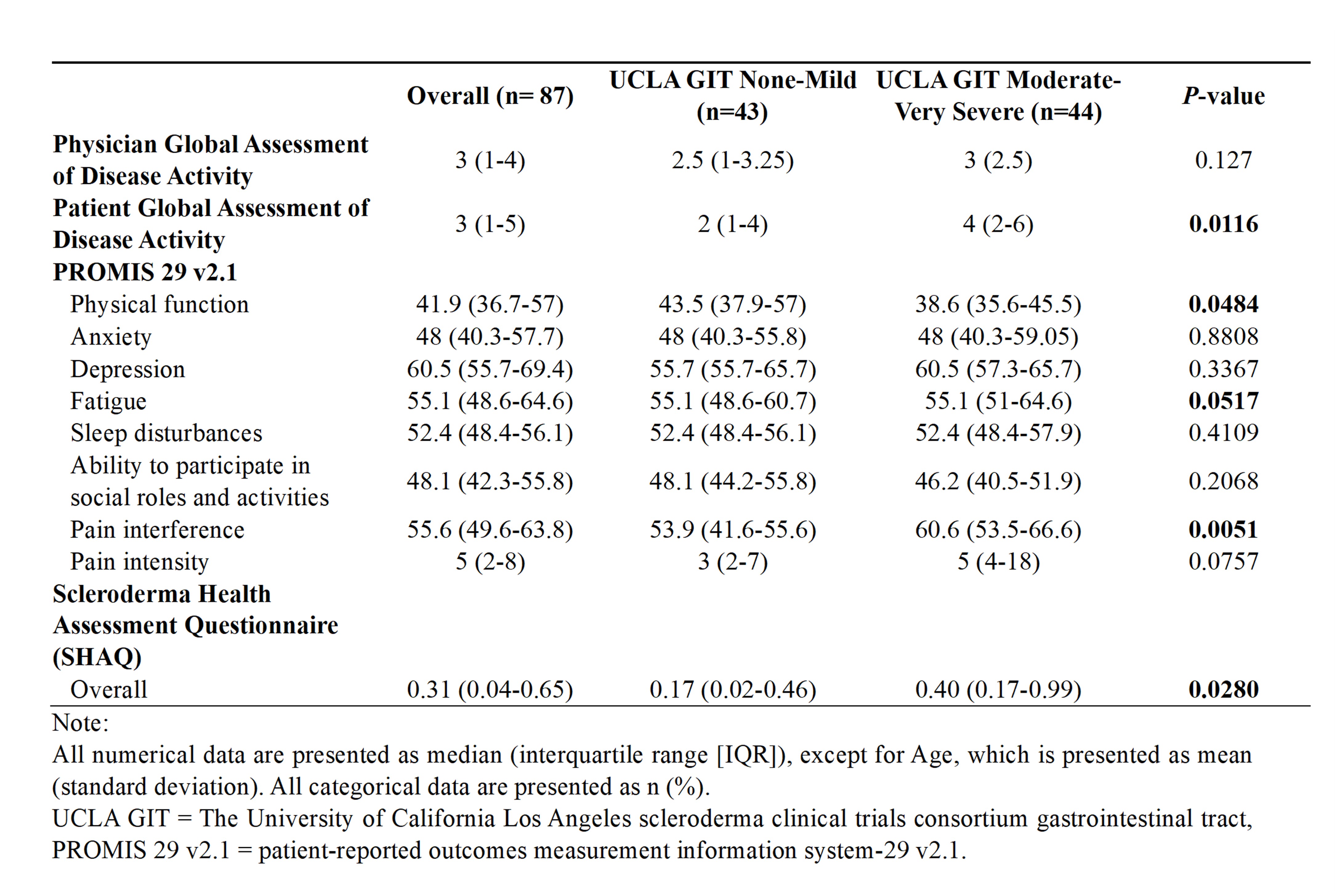
Results: 87 SSc patients (mean age 59.14 y ± 12.17, 84% females, 91% white) were enrolled in the study. (Table 1) Disease duration from the first Raynaud symptoms in the SSc group with none-mild GI symptoms was significantly longer than the group with moderate-severe GI symptoms (15 vs. 6 years, p=0.0009). Anxiety (6 vs. 4, p = 0.007) and PtGA of disease activity was higher (4 vs. 2, p=0.01) among those with moderate-severe GI symptom severity. Patients with mod-severe GI symptoms also exhibited worse lung disease severity (1 vs. 0, p = 0.04), worse physical function (38.6 vs 43.5, p=0.05), worse pain scores (60.6 vs. 53.9, p=0.005) and worse overall function and SHAQ (0.40 vs. 0.17, p=0.028). (Table 2) Patients with mod-severe GI symptoms also had higher bowel frequency (14 vs.10, p=0.0098) and looser stool consistency (4.73 vs. 3.91, p=0.0002). There was no significant difference in small bowel or colonic permeability between the two symptom severity groups in the subset of patients studied.
Conclusion: While SSc patients with higher UCLA GIT 2.0 symptom scores clearly demonstrate worse physical and mental health-related quality of life, our study did not find a significant difference in intestinal permeability in a limited subset of those with moderate-severe vs none-mild GI symptom severity. Pathogenetic mechanisms for GI involvement in SSc require further elucidation. Molecular and microbiome studies are currently underway.
To cite this abstract in AMA style:
Lesmana E, Keehn A, Karn A, Pauly A, Breen-Lyles M, Edwinson A, Grover M, Makol A. Gastrointestinal Symptom Severity and Intestinal Permeability in Systemic Sclerosis: A Single Center Prospective Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/gastrointestinal-symptom-severity-and-intestinal-permeability-in-systemic-sclerosis-a-single-center-prospective-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gastrointestinal-symptom-severity-and-intestinal-permeability-in-systemic-sclerosis-a-single-center-prospective-study/