Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Basic Science Poster
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Traditional studies of skin fibroblasts in SSc focus on the early “migratory” fibroblast population derived from explant culture of skin biopsies. Single-cell RNAseq has delineated multiple fibroblast subpopulations in healthy control (HC) and SSc skin. We sought to isolate the “resident” non-migratory fibroblast populations from HC and SSc skin using a novel cell isolation technique. These were compared using in vitro functional assays, and bulk RNAseq to delineate their fibrotic potential, and determine which clusters of fibroblasts, defined by scRNAseq of whole skin, were present in the resident or migratory populations and how they relate to different types of skin disease in SSc.
Methods: Forearm skin punch biopsies were collected from dcSSc (n=3) and HC (n=3). Migratory fibroblasts were isolated by standard explant culture. The remaining biopsy fragments underwent collagenase digestion to yield the resident fibroblast population.
Functional characterization included 3-D collagen gel contraction, migratory scratch-wound assays. Western blot compared expression of pro-collagen I, CTGF and αSMA. Bulk RNAseq was performed on each fibroblast population, with significant differences in gene expression defined as: fold change≥1.5, and adjusted p-value< 0.05.
scRNAseq was performed on 12 SSc whole skin biopsies, and 3 HC, using the 10X Genomics platform.
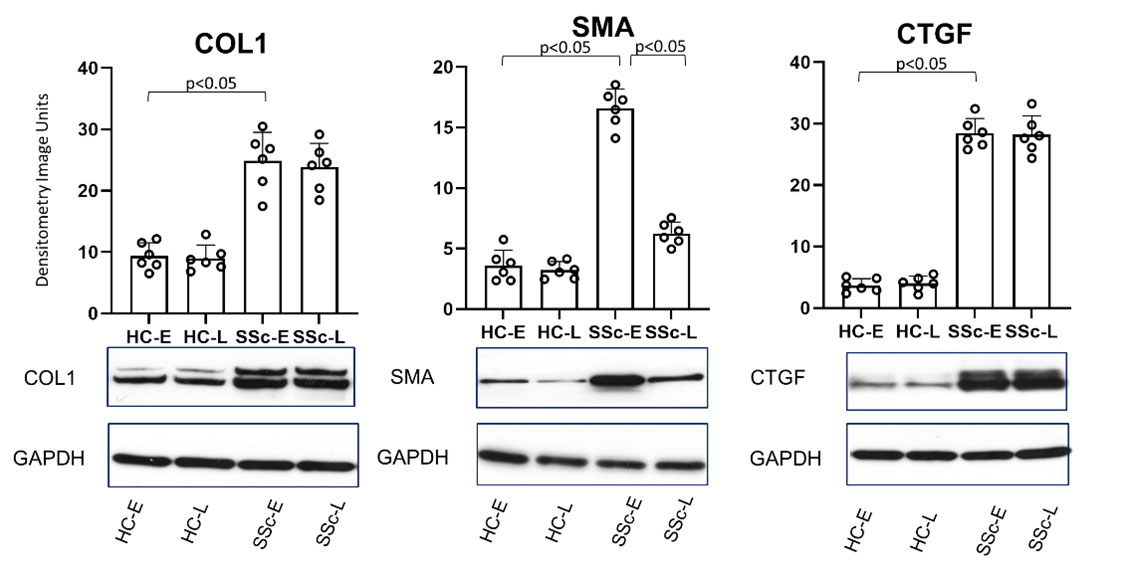
Results: SSc migratory fibroblasts showed a characteristic fibrotic phenotype with increased gel contraction, faster migration and overexpression of COL1, CTGF and αSMA compared to HC. However, the SSc resident fibroblasts showed lower expression αSMA compared to SSc migratory, but similar COL1 and CTGF expression (p< 0.05) (Figure1). Functional assays confirmed differences between SSc migratory, SSc resident and HC fibroblasts.
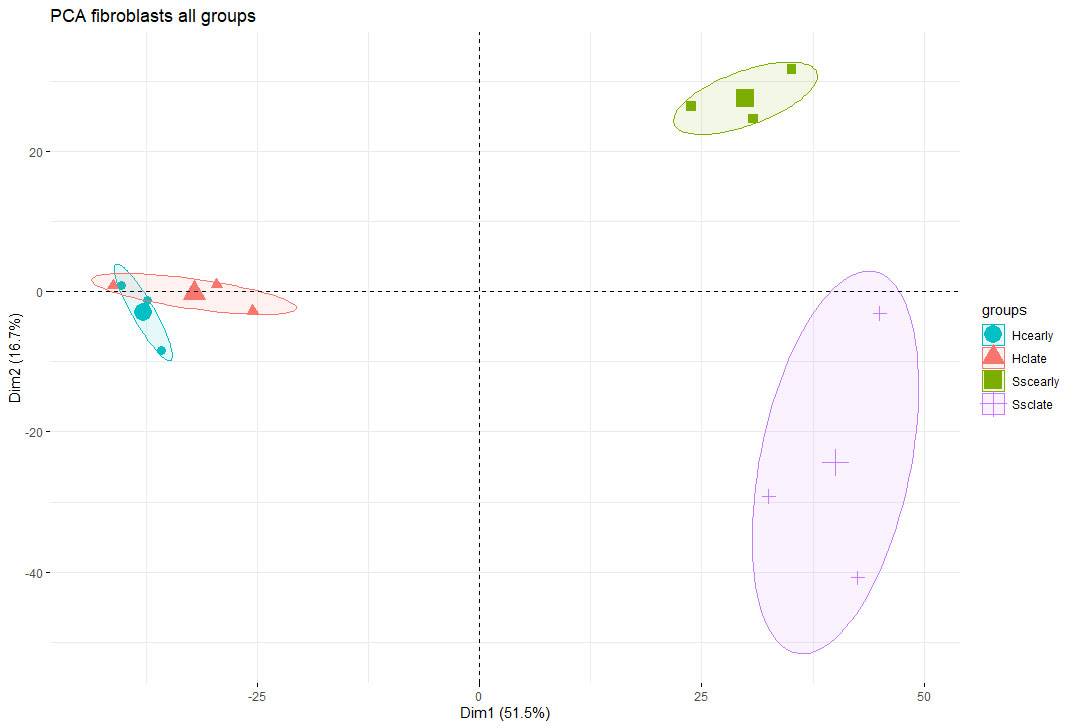
Principle component analysis confirmed clear separation of the SSc fibroblast populations (migratory and resident) from each other and fromnHC (Figure 2), with 1483 genes being significantly differentially expressed between the SSc fibroblast populations.
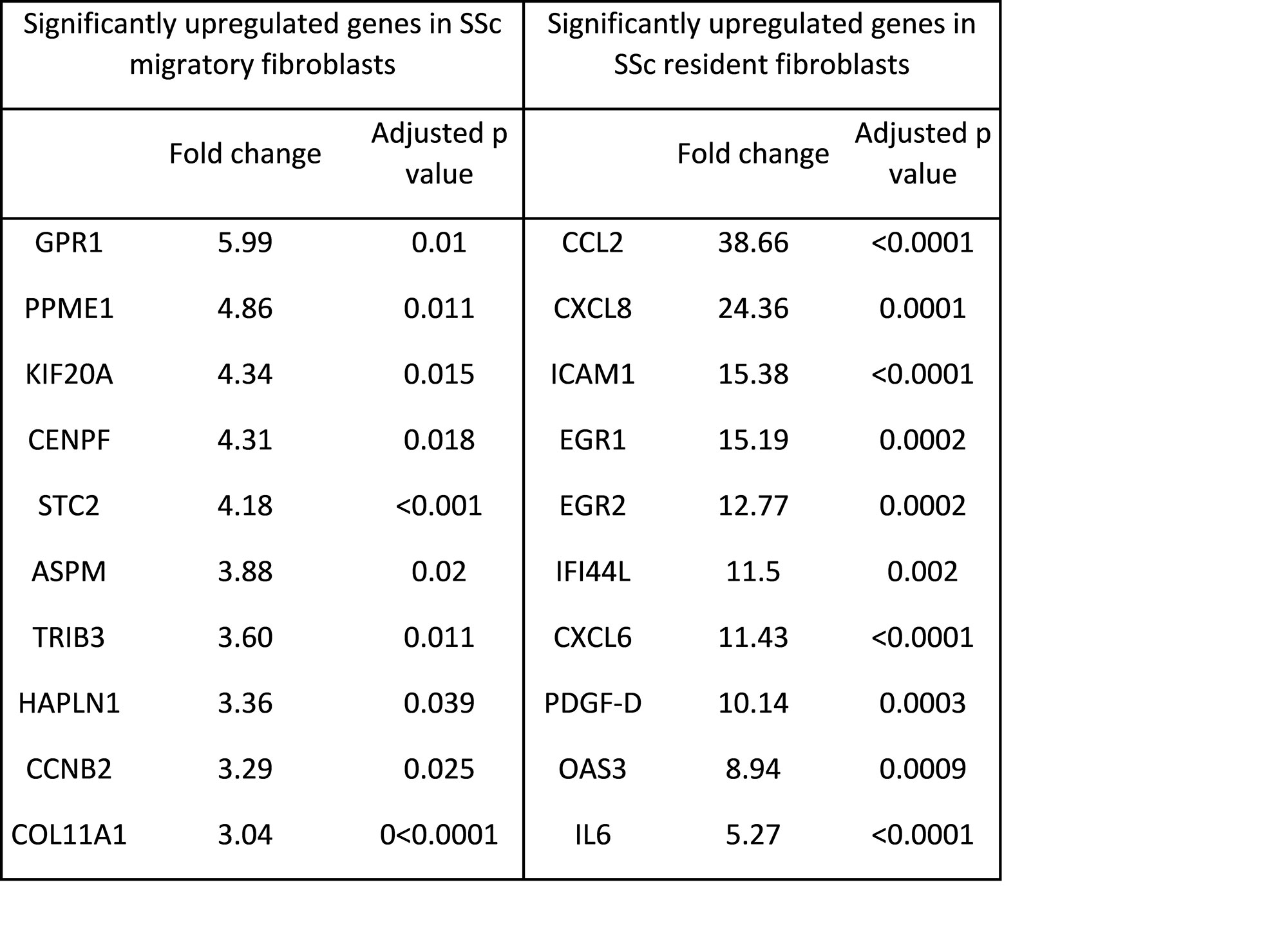
Performing scRNAseq, 10 clusters of fibroblasts were identified in skin. Of these, cluster 0 and 4 showed increased levels of COL11A1, STC2 and TRIB3 in keeping migratory fibroblasts. Increased levels of CCL2, ICAM1, IL6 were seen in clusters 3 and 6, representing similar gene expression pattern as resident fibroblast population (Table1).
Conclusion: We demonstrate that both SSc resident and migratory populations show a characteristic disease phenotype compared with HC. Interestingly gene expression profiles differ markedly between resident and migratory fibroblasts for SSc whereas no major differences were observed for HC. This suggest that these two fibroblast populations may each contribute differently to SSc pathogenesis, potentially mediating fibrotic versus inflammatory functions. Future work will focus on understanding their role in SSc pathogenesis, and their contribution to disease phenotype, potentially facilitating a more targeted treatment approach.
To cite this abstract in AMA style:
Clark K, Cole A, Xu S, Ong V, Buckley C, Denton C. Functionally Distinct Fibroblast Populations in Systemic Sclerosis Skin Revealed by Explant Tissue Characterization and Single-cell RNAseq [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/functionally-distinct-fibroblast-populations-in-systemic-sclerosis-skin-revealed-by-explant-tissue-characterization-and-single-cell-rnaseq/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/functionally-distinct-fibroblast-populations-in-systemic-sclerosis-skin-revealed-by-explant-tissue-characterization-and-single-cell-rnaseq/