Session Information
Date: Monday, November 14, 2022
Title: Abstracts: RA – Treatment III: Comorbidities and Consequences
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Recently, it has been recognized that frailty and pre-frailty are common in patients with rheumatoid arthritis (RA) [1]. Whether frailty status portends an increased risk of adverse outcomes in patients with RA on biologic or targeted synthetic disease modifying anti-rheumatic drugs (b- or tsDMARDs) remains unknown. Our objective was to evaluate the association between frailty and risk of infections in patients with RA exposed to b- or tsDMARDs.
Methods: Using the IBM/Watson MarketScan® Commercial Claims and Encounters Databases, we identified all patients with RA who filled new prescriptions (or received infusions) for TNFα antagonists (TNFi), non-TNFi biologics (rituximab, abatacept, tocilizumab) or Janus Kinase inhibitors (JAKi) between 2008-2019. The date of the first prescription within these three drug categories was the index date. Patients’ frailty risk score was calculated using the Claims-Based Frailty Index (CFI) [2, 3], which estimates a deficit-accumulation frailty index using International Classification of Diseases codes, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes in administrative claims data in the 1-year baseline period. The index ranges from 0 (not at all frail) to 1 (severely frail). The primary outcome was time to infections requiring hospitalization. Secondary outcomes included outpatient or inpatient encounters for infections and all-cause hospitalizations.
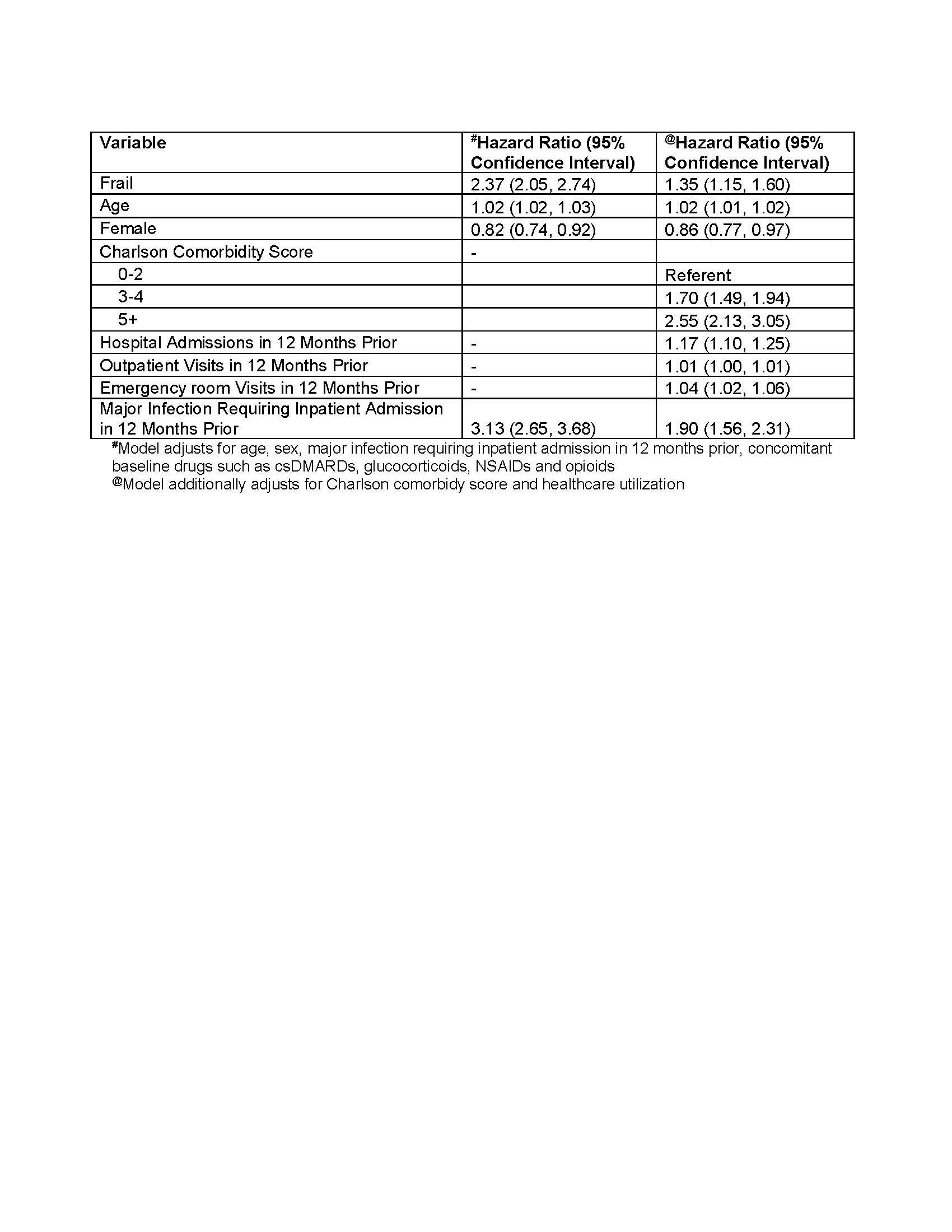
Patients were followed until 1) outcome occurrence; 2) disenrollment; 3) >90 days elapsed (or >180 days for rituximab) without further fills of the first drug categories; 4) they filled/received infusions of b/tsDMARDs from a different drug category; or 5) 2 years after index. We used Cox proportional hazards adjusting for demographics, calendar year, serious and/or opportunistic infections in the 12-months prior to index to estimate the adjusted hazard ratios (aHR) and 95% confidence intervals (95% CIs) for each outcome. In separate model, we additionally adjusted for comorbidity burden, and health care utilization (HCU).
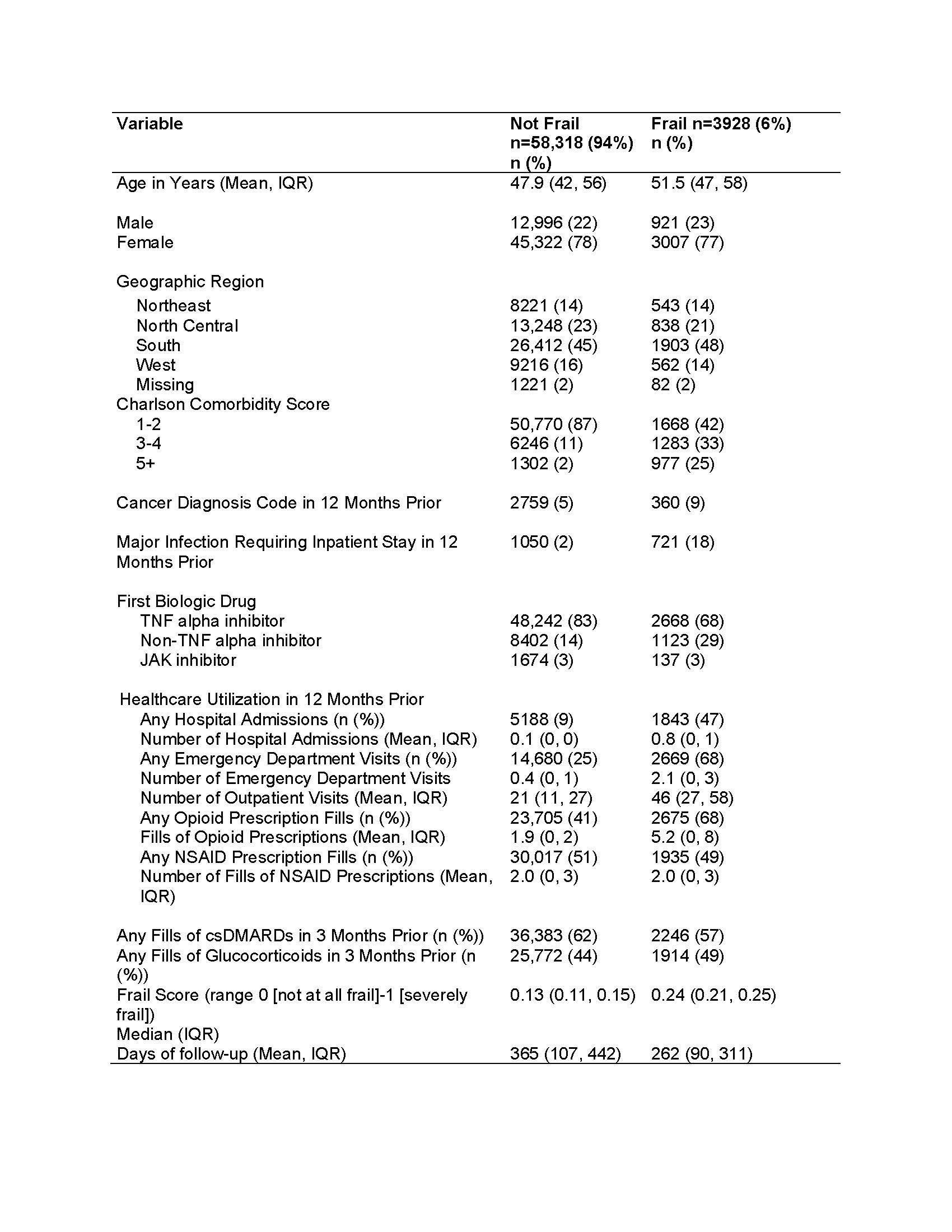
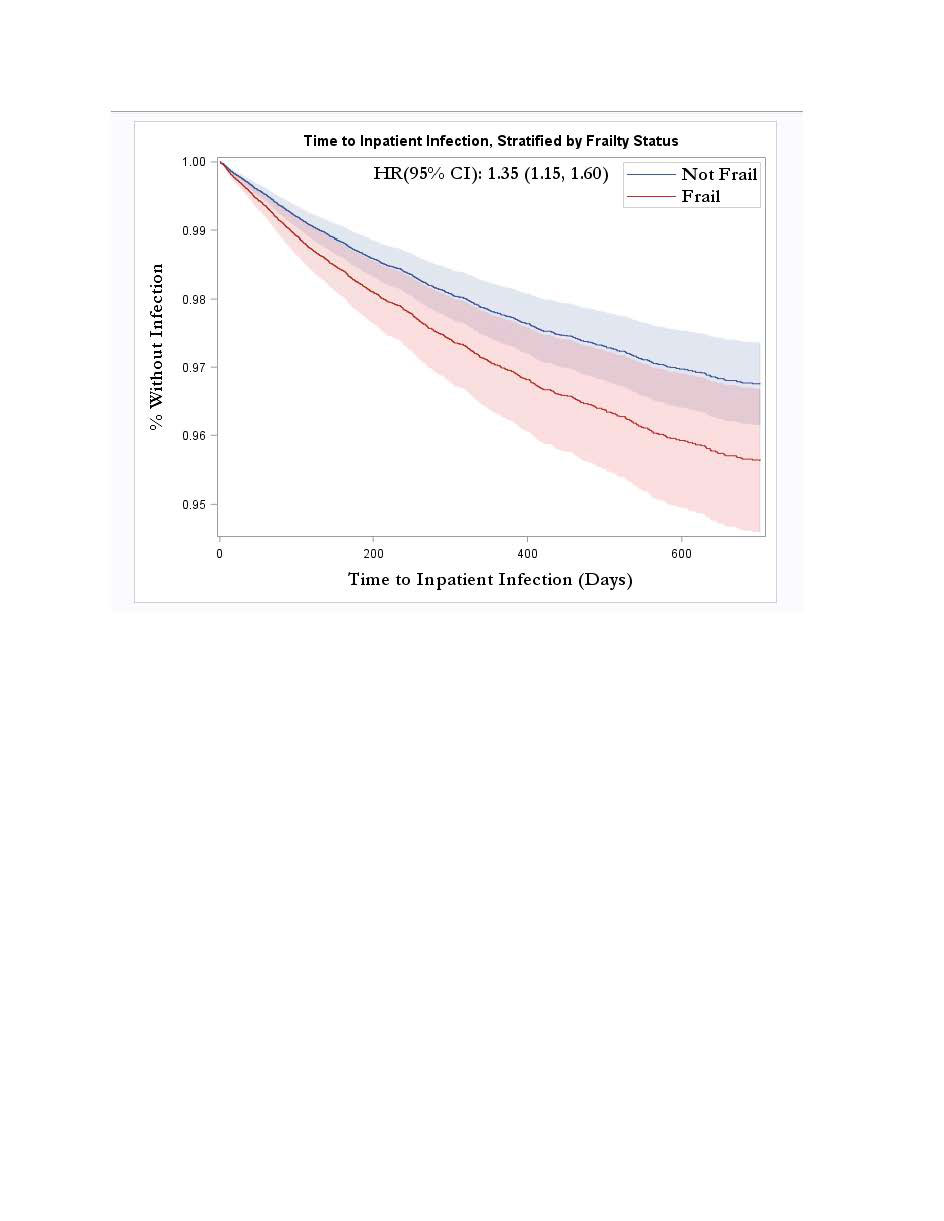
Results: A total of 62,246 patients with RA met our inclusion criteria of whom 50,910 (82%) started TNFi as their first biologic, 9525 (15%) non-TNFi biologics, and 1811 (3%) JAKi. Among these, 3928 (6%) were considered frail (Table 1). In multivariable analyses, frail patients had higher risk of serious infections compared to non-frail patients (aHR 2.37, 95% CI 2.05-2.74) which decreased to aHR 1.34, 95% CI 1.13-1.58 (Table 2, Figure 1) after adjusting for comorbidity burden and the HCU. Similarly, frailty was associated with increased risk of any infection (aHR 1.18, 95% CI 1.11-1.25), and all-cause hospitalizations (aHR 1.34, 95% CI 1.21-1.49) relative to non-frail individuals.
Conclusion: Frailty is an important predictor for the risk of adverse outcomes among patients with RA treated with b- or tsDMARDs. Our findings underscore the need for considering this parameter in patient evaluations (even among younger patients) in the clinic.
Abbreviations: csDMARD: conventional synthetic disease modifying anti-rheumatic drugs; IQR: Interquartile range; NSAID: Non-steroid anti-inflammatory drug; TNF: Tumor necrosis factor
To cite this abstract in AMA style:
Singh N, Gold L, Wysham K, Andrews J, Reid P, Makris U, England B, Lee J, George M, Baker J, Jarvik J, Heagerty P, Singh S. Frailty Is Associated with Serious Infections in Biologic and Targeted-synthetic DMARD Treated Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/frailty-is-associated-with-serious-infections-in-biologic-and-targeted-synthetic-dmard-treated-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/frailty-is-associated-with-serious-infections-in-biologic-and-targeted-synthetic-dmard-treated-patients-with-rheumatoid-arthritis/