Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: There is increasing interest in the safety of COVID-19 vaccination in patients with underlying chronic conditions. We investigated the rates of side effects and/or disease flares in the short term after COVID-19 vaccination in patients with rheumatic diseases.
Methods: In this single-center observational study, we analyzed the proportion of self-reported systemic side effects and flares in patients with rheumatic diseases who received one of the approved COVID-19 vaccines in our country (BNT162b2, AZD1222, mRNA-1273 and JNJ-78436735) between Dec. 27, 2020 and May 28, 2021. Demographic data, type of rheumatic disease, activity of the disease determined by clinical scores when applicable, treatment, and previous SARS-CoV-2 infection were also collected.
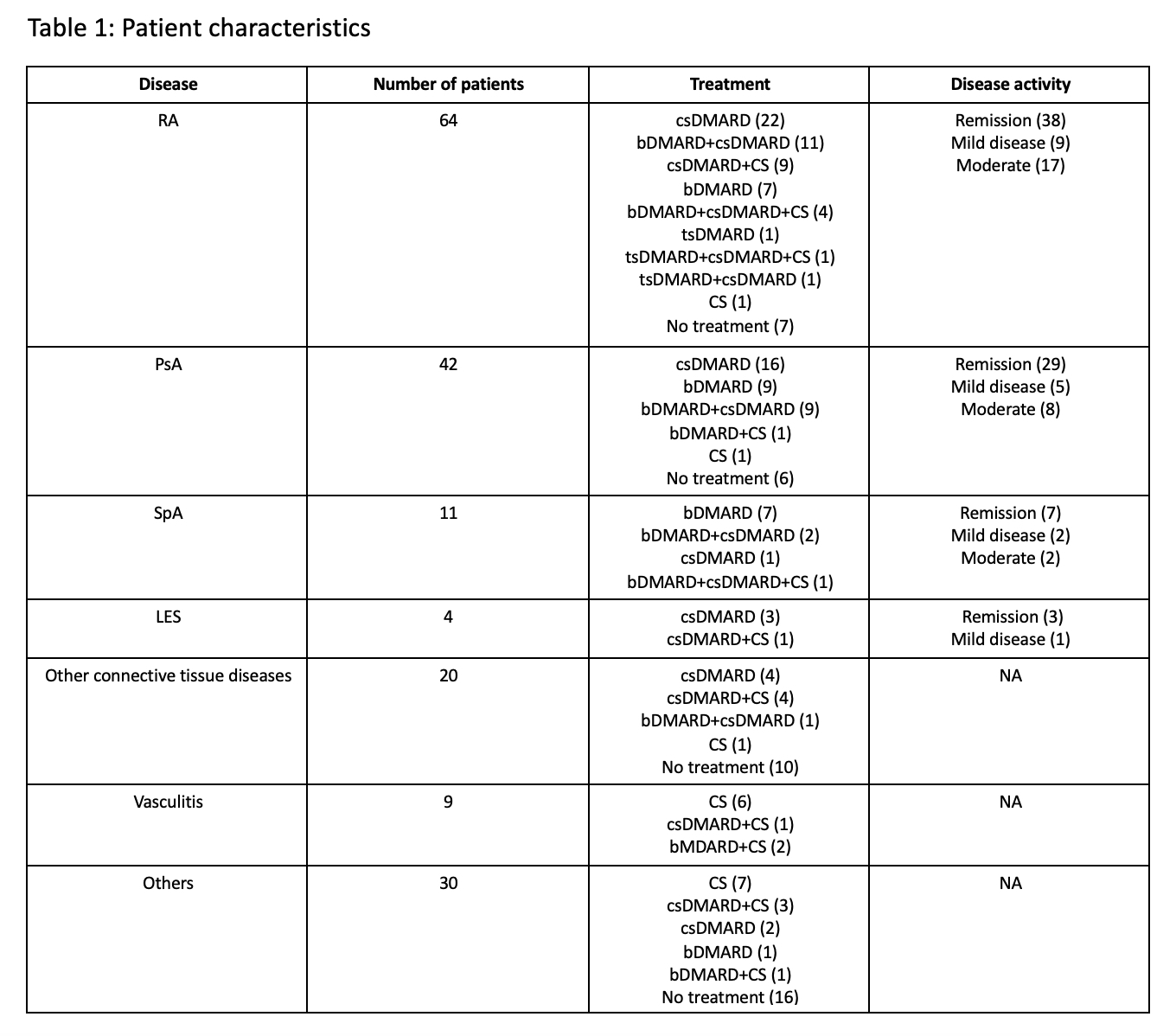
Results: One-hundred and eighty patients (female=127) were included in the study. Median age was 70 years (range 23-92), due to the fact that our vaccination campaign began with older and fragile patients. Patient characteristics are summarized in Table 1.
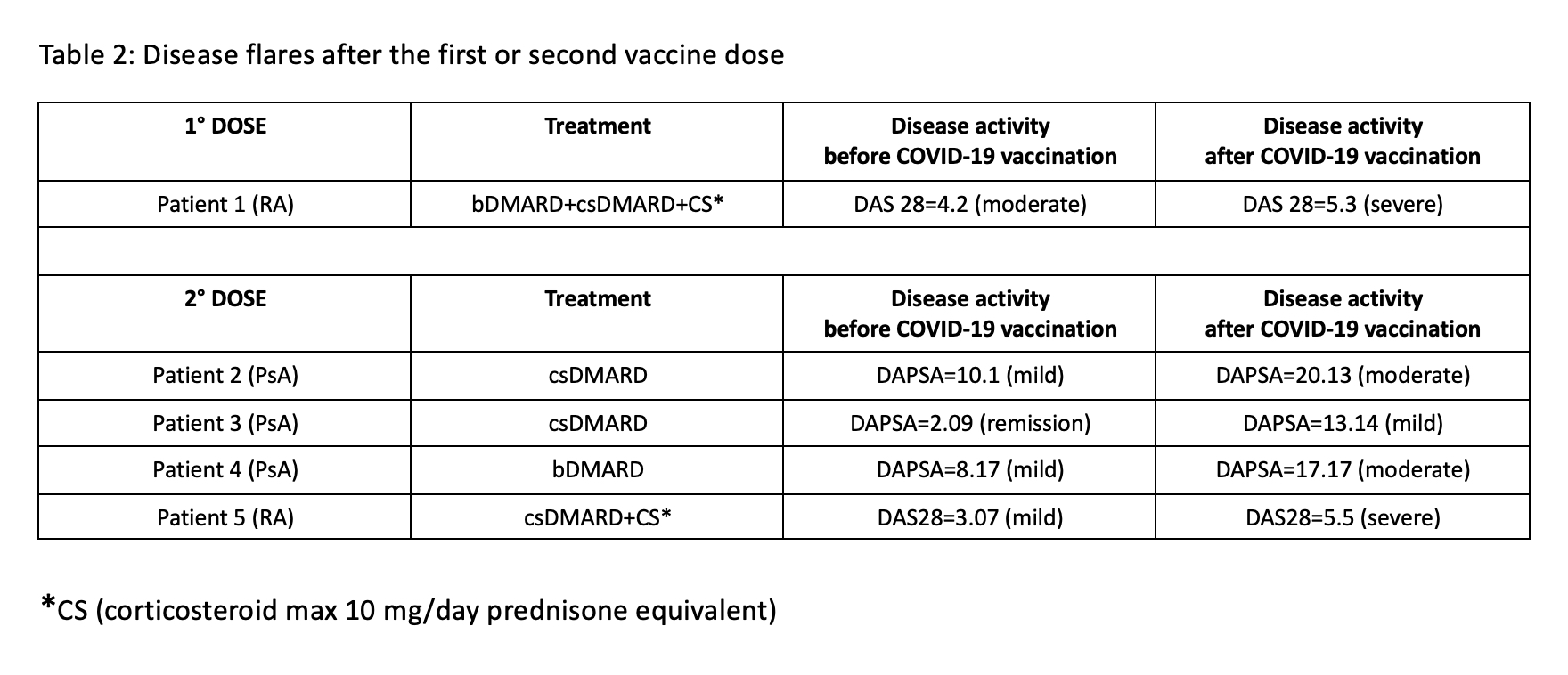
Of the 180 patients, 146 received BNT162b2, 25 AZD1222, 8 mRNA-1273, and 1 patient received JNJ-78436735. Six patients reported SARS-CoV-2 infection prior to vaccination. Thirty-four patients (18.9%) reported systemic side effects after the first dose: BNT162b2 23/146 (15.7%); AZD1222 10/25 (40%); mRNA-1273 1/8 (12.5%); JNJ-78436735 0/1. A total of 118 patients had received the second dose of the BNT162b2 or mRNA-1273 vaccine at the time of data collection. Of these, 39 (33.1%) reported systemic side effects after the second dose: BNT162b2 38/112 (33.9%); mRNA-1273 1/6 (16.7%). The most frequent side effects were fever, headache, fatigue and arthromyalgia. All systemic side effects were mild and resolved spontaneously within a few days. Disease flare was reported in 1 out of the 180 patients (0.6%) who received the first dose, and in 4 out of the 118 patients (3.4%) who received the second dose. Two of these five patients had RA and three had PsA. All five had received BNT162b2. These results are illustrated in Table 2.
Conclusion: Preliminary results suggest that disease flares are possible but infrequent after COVID-19 vaccination in patients with rheumatic diseases. Rates of systemic side effects are comparable to the general population. Further studies are needed with a larger number of patients with rheumatic diseases to better understand this phenomenon and possibly identify predictive factors for disease flare.
To cite this abstract in AMA style:
Carbone A, Vukatana G, Vandelli E, Trevisani M, Rossi E, Mulè R, Fusconi M. Flares and Side Effects After COVID-19 Vaccination in Patients with Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/flares-and-side-effects-after-covid-19-vaccination-in-patients-with-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/flares-and-side-effects-after-covid-19-vaccination-in-patients-with-rheumatic-diseases/