Session Information
Date: Sunday, November 12, 2023
Title: (0283–0307) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Idiopathic inflammatory myopathies (IIM, myositis) are a systemic autoimmune disease leading to debilitating muscle weakness and significant limitations in daily activities. Physical activity monitor (PAM) are validated and recognized measures of the frequency and intensity of physical activities. Given the urgent need for objective, continuous, longitudinal outcome measures of physical activity and function in myositis, we assessed compliance, reliability, and validity of widely used commercial PAM – Fitbit© in evaluating physical activity in myositis patients.
Methods: “Myositis Patient Centered Tele-Research (MyPACER)” is a multi-center observational prospective study conducted over 6 months. The study had two cohorts, 1. Tele-Research Cohort (TRC): patients were remotely enrolled from any location in the United States, and 2. Center-Based Cohort (CBC): a traditional cohort with enrollment from the 2 myositis centers. Functional and Patient-Reported Assessments were completed monthly, including a health assessment questionnaire, patient global disease activity, PROMIS-physical function 20 and functional tests timed up-and-go (TUG), and sit-to-stand (STS)). Participants were asked to use their waist-based Fitbit (≥10 hours/day), ≥ 7 days/month. Average steps per minute & Average peak cadence were evaluated using Fitbit data.
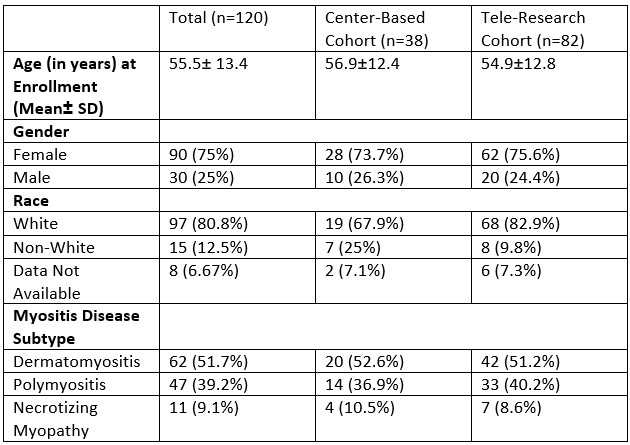
Results: 120 IIM patients (mean age 55.5+/- 13.43, 75% females, 80.8% white), who completed baseline visit were enrolled in the study, comprising 82 in the Main TRC group, 21 in Local TRC and 17 in the local CBC (table 1). There were 51.7% DM, 39.2% PM, and 9.1% NM. The TRC and CBC cohorts were similar in demographics and disease subtypes. Age was significantly correlated with the average steps per minute (Ave Step/min) (p=0.01) but not with ave. peak cadence, showing a decrease in steps with advancing age. Gender, race/ethnicity or disease subtypes were not associated with PAM measures.
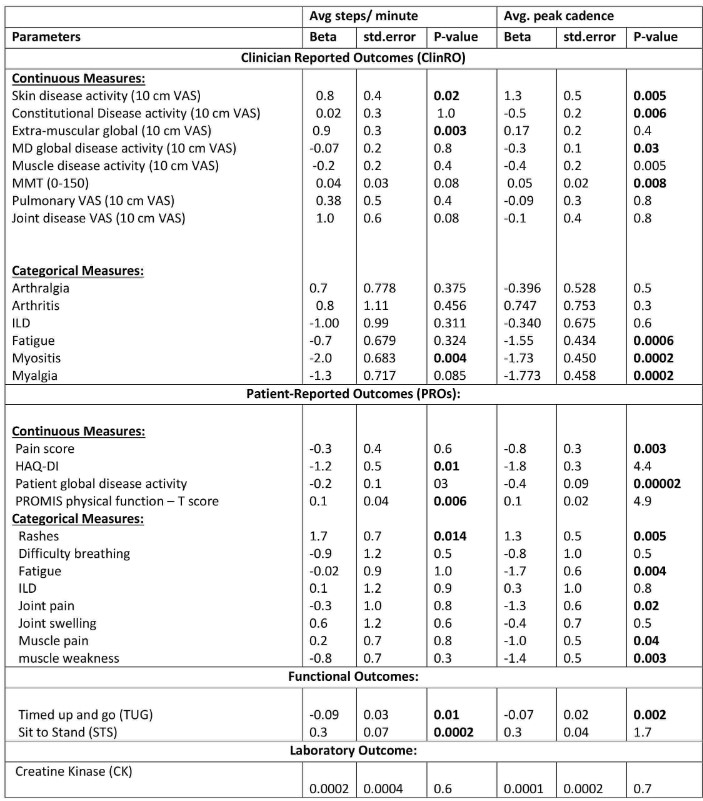
On data analysis, 90% patients completed at least one valid day on their Fitbit devices. The compliance with Fitbit was very high with participants wearing devices on most days of the week (Ave. = 6.52 days) for the most visits (Ave. 5.73 visits), with similar results for remote or local recruitment. Ave steps per minute and peak cadence showed strong test-retest reliability (r=0.89 and r=0.86, p< 0.0001 & p= 0.0001) and were strongly correlated within-patient. Regarding validity at baseline, ave steps/mins was significantly associated with myositis, skin disease, extra-muscular global, PROMIS physical function and functional tests, HAQ score but not with other myositis core set measures (Table 2).While the ave. peak cadence was significantly associated with MMT-8, physician and patient global disease activity as well as fatigue, myalgia, myositis, TUG, but not with HAQ, CK, and extra-muscular disease activity (Table 2).
Conclusion: In a large IIM cohort, Fitbit physical activity monitor variables demonstrates favorable compliance and psychometric properties with a strong test-retest reliability and association of several patient and physician-reported measures.
To cite this abstract in AMA style:
Sharma A, keret s, Lomanto Silva R, Chandra T, Levin J, Moghadam-Kia S, V. Oddis C, Aggarwal R. Fitbit Is a Valid and Reliable Physical Activity Monitor in Idiopathic Inflammatory Myopathy [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/fitbit-is-a-valid-and-reliable-physical-activity-monitor-in-idiopathic-inflammatory-myopathy/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fitbit-is-a-valid-and-reliable-physical-activity-monitor-in-idiopathic-inflammatory-myopathy/