Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL) is a potent oral selective janus kinase 1 inhibitor which is currently being investigated as an agent to treat rheumatoid arthritis (RA). In the FINCH 1 study (NCT02889796), FIL in combination with methotrexate (MTX) provided a rapid and meaningful clinical response in patients with inadequate response to MTX. Chronic inflammatory diseases such as RA substantially affects a broad range of aspects of the patient’s health related quality of life including work participation.1 The aim of this post-hoc analysis was to evaluate the rate and magnitude of change from baseline and assess the effect of treatment response on work productivity and activity scores in RA patients from the FINCH 1 study.
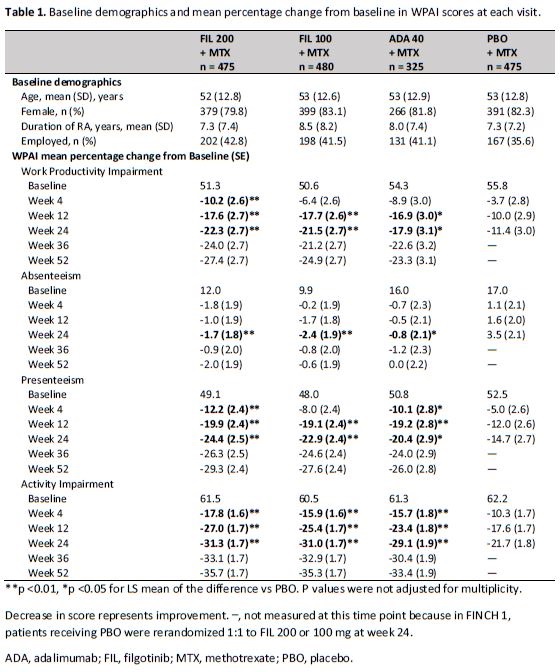
Methods: Patients with active RA were randomized to receive FIL 200 mg (n=475), FIL 100 mg (n=480), or subcutaneous adalimumab (ADA) 40 mg (n=325), or PBO (n=475) with concomitant MTX treatment in all groups for up to 52 weeks. At week 24, patients receiving PBO were randomized 1:1 to FIL 200 mg or 100 mg. The activity impairment, work productivity impairment, presenteeism, and absenteeism in the past 7 days were evaluated at baseline and then at weeks 4, 12, 24, 36 and 52 on treatment, using the self-administered 6-item Work Productivity and Activity Impairment Questionnaire: Rheumatoid Arthritis (WPAI:RA). (employed patients only) The 4 subscale scores ranged from 0%-100%, with higher scores indicating greater impairment. changes from baseline in WPAI: RA score were analyzed using mixed effect models for repeated measures (MMRM), and missing data were not imputed. All analyses were exploratory without multiplicity adjustment, and nominal P values are reported.
Results: The baseline demographics were relatively consistent across all treatment arms. Patients were (mean + SD) 35 + 13 years old, 82% female and 40% employed. Statistically significantly greater improvement in WPAI scores from baseline compared to PBO was reported as early as week 4 with FIL 200 mg, and week 12 with FIL 100 mg for work productivity, presenteeism, and activity (Table 1). At 24 weeks, both doses of FIL resulted in 21.5-24.4% improvement in work productivity and presenteeism compared to 11.4-14.7% in PBO. Similarly, both doses of FIL improved activity impairment score by 31% from baseline compared to 21.7% in PBO. Improvement over baseline persisted throughout treatment until week 52 for both FIL doses. WPAI scores were similar for both FIL doses and ADA throughout the study.
Conclusion: Improvement in overall work productivity, presenteeism, and daily activity was greater with both doses of FIL in combination with MTX compared to PBO + MTX in RA patients who had inadequate response to MTX.
Reference:
- Kim et al. J Rheumatol. 2017;44(8):1112-1117.
To cite this abstract in AMA style:
Younossi Z, Stepanova M, Gerber L, Lee S, Hu H, Hendrikx T, Boonen A, Walker D, Alten R, Combe B. Filgotinib Improved Work Productivity and Activity Impairment in Patients with Rheumatoid Arthritis and Inadequate Response to Methotrexate: Results from FINCH-1 Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/filgotinib-improved-work-productivity-and-activity-impairment-in-patients-with-rheumatoid-arthritis-and-inadequate-response-to-methotrexate-results-from-finch-1-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/filgotinib-improved-work-productivity-and-activity-impairment-in-patients-with-rheumatoid-arthritis-and-inadequate-response-to-methotrexate-results-from-finch-1-study/