Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders III: Clinical Trials
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Lower gastrointestinal tract (GIT) complications are common in patients with systemic sclerosis (SSc), associate with a high disease burden, and current treatment alternatives are limited. Patients with SSc have also an altered intestinal microbiota composition. This provides a rational for the investigation of fecal microbiota transplantation (FMT) in SSc patients with lower GIT symptoms. In this randomized mutlicenter double-blind clinical trial (RCT) we assessed the safety and efficacy of a standardized intestinal microbiota infusion in SSc patients with lower GIT symptoms.
Methods: Patients with SSc and moderate to severe bloating and/or diarrhea assessed by the UCLA SCTC GIT score 2.0 were enrolled in a multicenter, double-blind, randomized, placebo-controlled phase 2 trial. Patients were randomized to receive FMT with an intestinal infusion of a standardized fecal microbiota culture (ACHIM) or placebo at weeks 0 and 2. At week 12, all patients received a FMT infusion and were followed in an open lable phase until week 20. The primary outcome was change between baseline and week 12 in UCLA GIT score item diarrhea or bloating measured as the average marginal effect (AME), depending on which was the worst symptom at baseline; evaluated separately for each patient. Comprehensive predefined subgroup analyses were conducted. Secondary outcomes were safety and tolerability and total UCLA GIT score. Other outcome measures included the change in UCLA GIT score from week 12 to week 20.
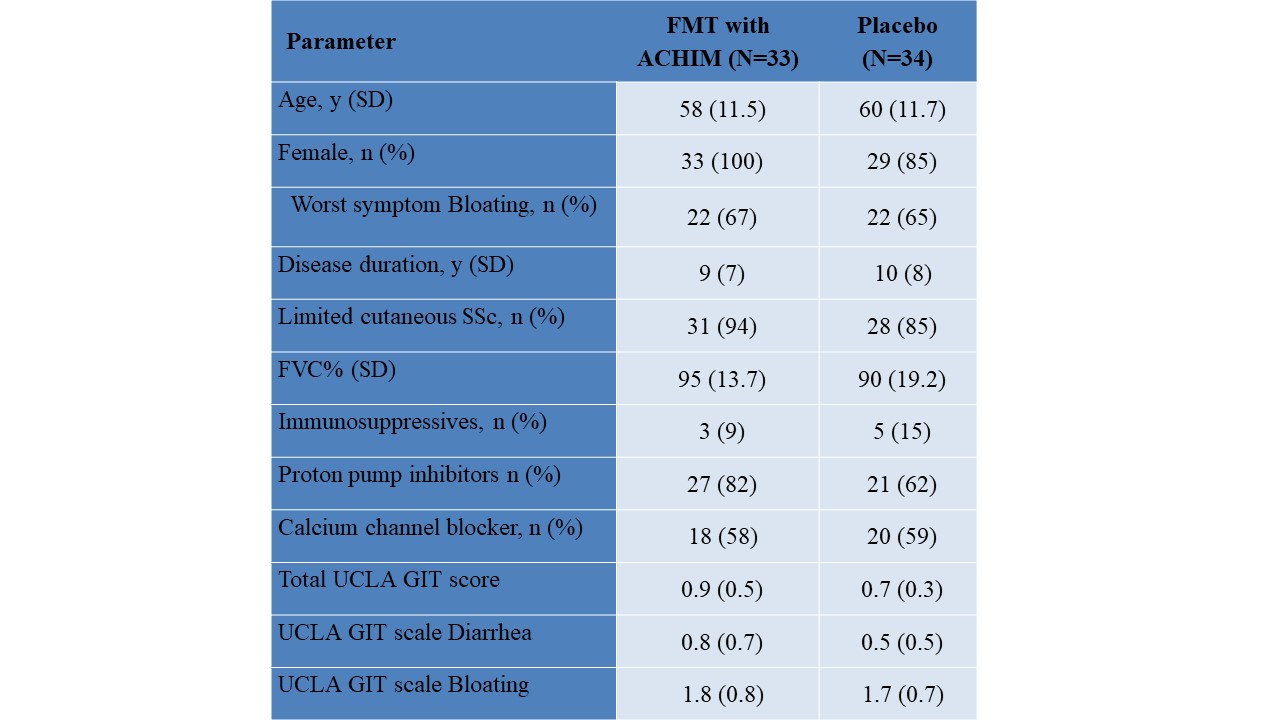
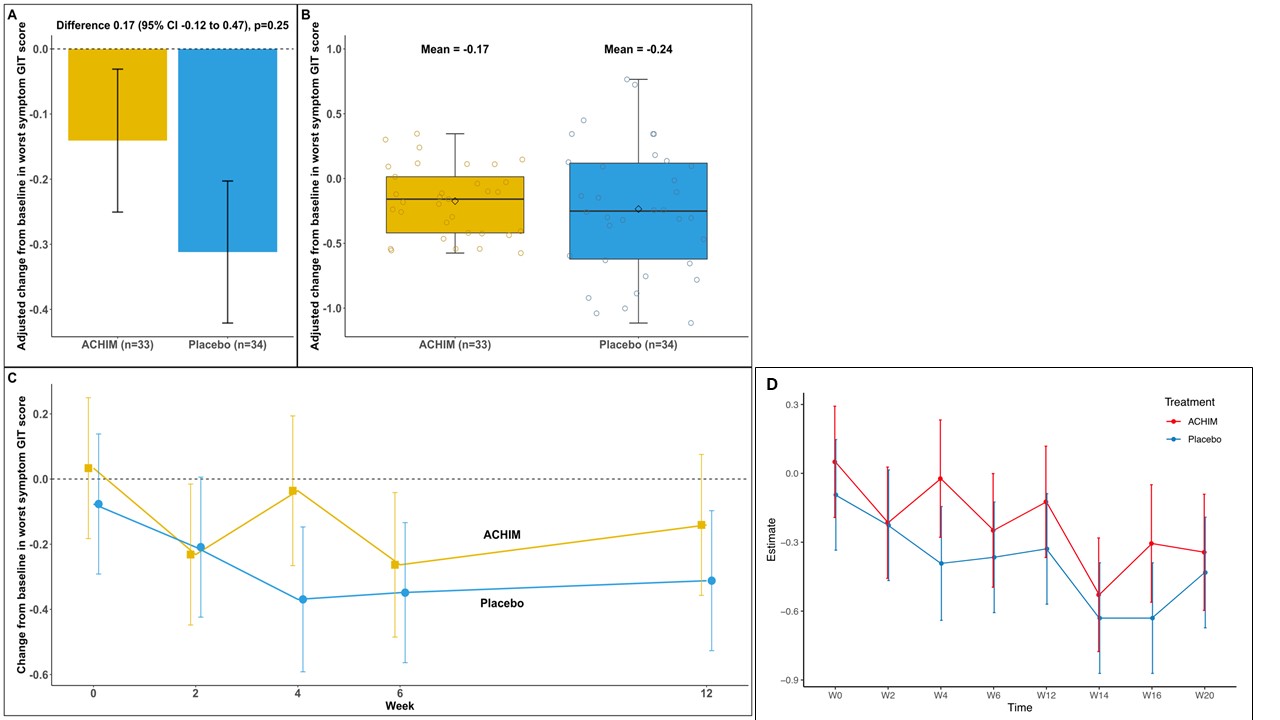
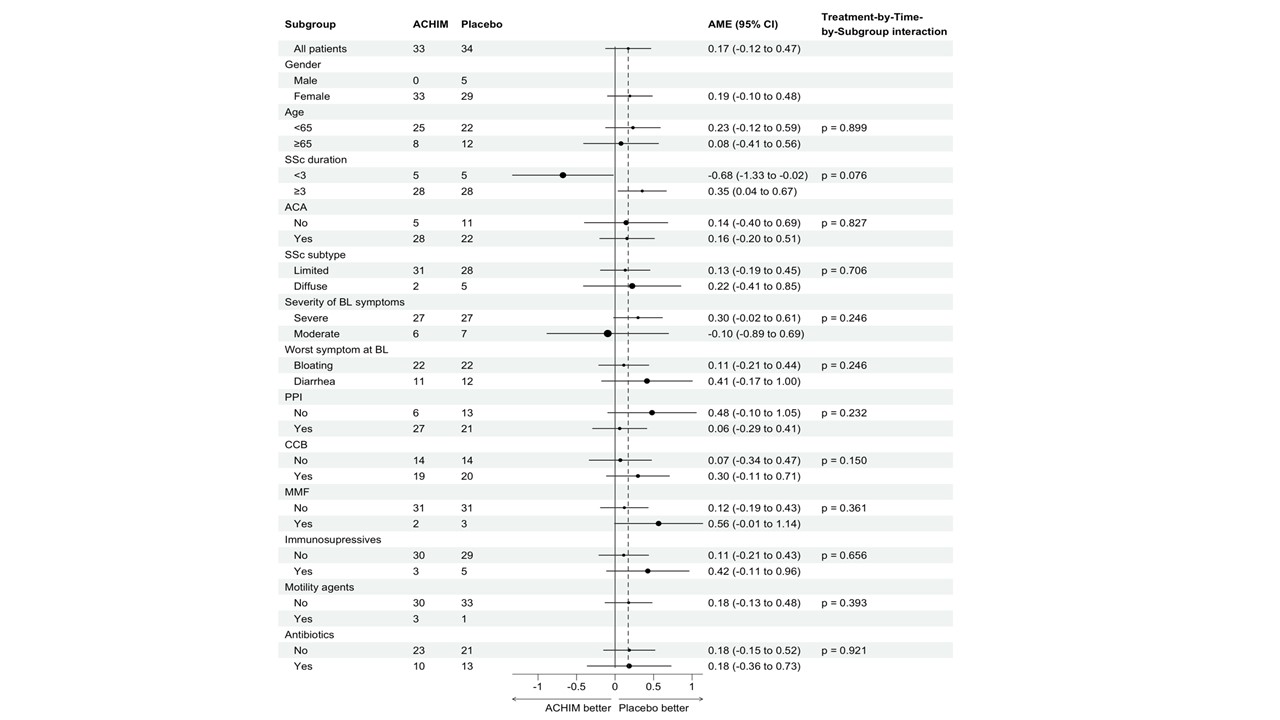
Results: A total of 65 patients were randomized to receive FMT or placebo. Baseline characteristics in the groups were comparable (Table). There was no significant difference in the change in lower GIT symptoms from week 0 to week 12 between the groups (AME=0.17 (-0.12, 0.47), p=0.25) and no significant differences in any of the predefined subgroup analyses (Figure 1A-C and 2). Similarly, no significant difference was observed in the total GIT score between the two groups (AME=0.09 (-0.04, 0.23), p=0.17). Furthermore, during the open lable period, there was no statistical difference in change between the ACHIM and the placebo group (AME= -0.04 (-0.32, 0.23), p=0.077) shown in Figure 1D. Participants treated with ACHIM, 16 (37%) and with placebo 19 (42%) experienced any side effects. These were in general mild and short-lasting, with abdominal pain as the most frequent side effect present in 5 (15%) in ACHIM and 2 (6%) in placebo. Time to resolved pain was 2days in both groups. One patient experienced an intramural perforation during gastroscopy and needed IV antibiotics but fully recovered.
Conclusion: We were unable to find indications that FMT improves lower GIT symptoms in SSc patients, but the treatment was found to be safe.
To cite this abstract in AMA style:
Hoffmann-Vold A, Fretheim H, Barua I, Nordgård Carstens M, Didriksen H, Sarna V, Lundin K, Distler O, Khanna D, Volkmann E, Midtvedt O, Midtvedt T, Dhainaut A, Halse A, Bakland G, Olsen I, Pesonen M, Molberg O. Fecal Microbiota Transplantation in Patients with Systemic Sclerosis and Lower Gastrointestinal Tract Symptoms: Data from the ReSScue Phase 2 Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/fecal-microbiota-transplantation-in-patients-with-systemic-sclerosis-and-lower-gastrointestinal-tract-symptoms-data-from-the-resscue-phase-2-randomized-clinical-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fecal-microbiota-transplantation-in-patients-with-systemic-sclerosis-and-lower-gastrointestinal-tract-symptoms-data-from-the-resscue-phase-2-randomized-clinical-trial/