Session Information
Date: Monday, November 18, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
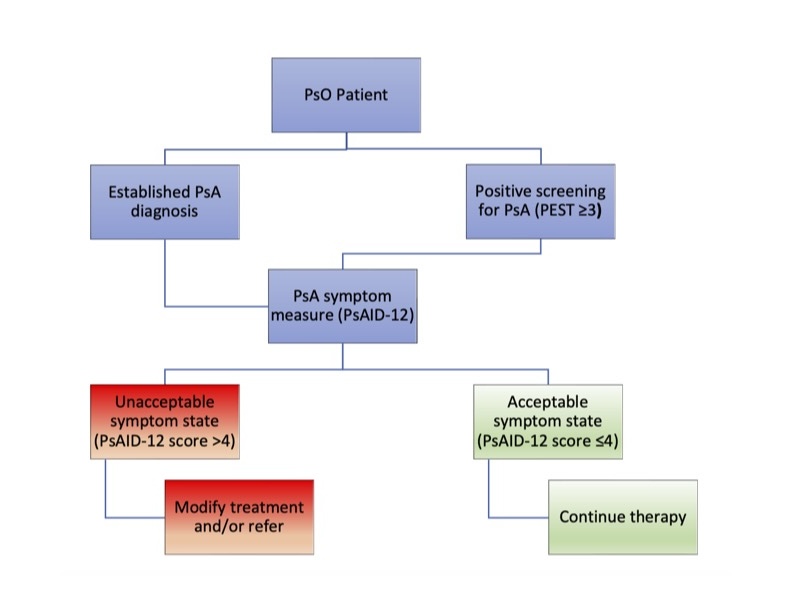
Background/Purpose: Psoriatic arthritis (PsA) is frequently undiagnosed and/or undertreated. Given the elevated risk of disability with treatment delays, we launched a quality improvement initiative to streamline PsA screening, symptom severity/impact assessment, and specialist referral to improve patient outcomes.
Methods: Following an established clinical framework, our study integrated the Psoriasis Epidemiology Screening Tool (PEST) and the Psoriatic Arthritis Impact of Disease 12-item questionnaire (PsAID-12) into the electronic medical record across 26 dermatology clinics. Patients with psoriasis (PsO) underwent PsA screening via the PEST. Those scoring ≥3 or already diagnosed with PsA subsequently completed the PsAID-12, which served to inform management. Applying the validated Patient Acceptable Symptom State (PASS) cutoff, a PsAID-12 score ≤4 is considered an acceptable PsA symptom state and supported continuation of current treatment. Conversely, a score above 4 indicated an unacceptable PsA symptom state, warranting treatment modification or referral to rheumatology. Results were sent to providers in real-time for review.
Results: Over 20 months, 7,140 patients with PsO were encountered by dermatology providers, with 1,159 (16.2%) having a baseline diagnosis of PsA. In total, 2,551 PsO patients completed the PEST; 652 (25.6%) scored ≥3 and subsequently completed the PsAID-12. Of those who took the PsAID-12, 63.4% scored ≤4, reflecting effective symptom management. Of the 36.6% of patients not on target, 80 (24.4%) received rheumatology referrals.
Conclusion: These results underscore the feasibility of our framework in facilitating PsA detection and assessment, referring patients to rheumatology, and ultimately improving the quality of care.
To cite this abstract in AMA style:
Ball G, Hamade H, Romanelli S, Zundell M, Shin S, Senthilkumaran T, Lamb A, Khattri S, Perez Chada L, Merola J, Gottlieb A. Feasibility of a Streamlined Approach for Psoriatic Arthritis Screening and Symptom Assessment [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/feasibility-of-a-streamlined-approach-for-psoriatic-arthritis-screening-and-symptom-assessment/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/feasibility-of-a-streamlined-approach-for-psoriatic-arthritis-screening-and-symptom-assessment/