Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Synovial monocytes and macrophages are heterogenous populations. These populations play diverse roles in the development of arthritis in humans and mice. In recent years, our laboratory identified a new synovial cell type we named tissue-resident monocyte-lineage cells (TR-MCs), which are are necessary for the development of inflammatory arthritis in mice. Although TR-MCs are CD64 negative and transcriptionally distinct from circulating monocytes and DCs, the current lack of targeted mouse models limit our ability to study this critical population and differentiate from other synovial monocyte and macrophage populations.
Methods: To study each synovial monocyte and macrophage population, we performed fate-mapping with 7 reporter models including Cx3cr1-ERcre-Ai3-YFP, Cx3cr1-Cre-Ai3-YFP, Ms4a3-cre-Ai3-YFP, P2ry12-ERcre-Ai3-YFP, Tmem119-GFP, Cd68-eGFP, and Csf1r-cre-Ai3-YFP in 8–12-week-old C57BL/6 mice. We also further characterized synovial monocyte and macrophage cells by performing cellular indexing of transcriptomes and epitopes (CITE-seq) on synovial macrophages (CD45+CD11b+CD4-CD8-Ly6G-SiglecF-NK1.1-CD64+) and monocytes (CD45+CD11b+CD4-CD8-Ly6G-SiglecF-NK1.1-MHCII-CD64-).
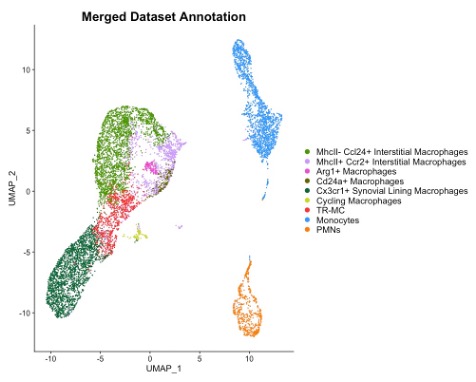
Results: Although 90% of synovial macrophages, 82% NCM and 76% TR-MC are derived from Cx3cr1-positive progenitor cells, only 41%, 91%, 27% of them express Cx3cr1 at 8-12 week-old. Although high percentages of Csf1r-expression were found in almost all synovial cell types, synovial macrophages express the highest intensity of Csf1r. Similarly, Ms4a3 was expressed at a high percentage in both granulocytes and monocyte-lineage cell, but the intensity of Ms4a3 is more than 2 times higher in macrophages than other cell types. P2ry12 was expressed in synovial macrophages and TR-MC with 20% positivity. The expression of CD68 and Tmem119 are same in all synovial myeloid cell types. To develop specific reporter models of synovial monocyte and macrophage subpopulations, we characterize them by merging the 2 CITE-seq datasets and identified 5 synovial macrophages subpopulations – MHCII+ CCL24+ interstitial macrophages, MHCII-CCR2+ interstitial macrophages, Arg1+ macrophages, CD24a+ macrophages and CX3CR1+ synovial lining macrophages as well as TR-MCs, monocyte-lineage cells and polymorphonuclear leukocytes (PMNs). MHCII- CCL24+ interstitial macrophages specifically expressed Cd209d and Ccl24 genes. Arg1+ macrophages exclusively expressed Arg1 and Flt1 genes. The differentially expressed genes of CX3CR1+ synovial lining macrophages are aligned with the CX3XR1+ lining macrophages published by Culemann S, et. al. CD24a+ macrophages highly expressed Tnip3 and Anxa8 genes as well as CD11c surface marker.
Conclusion: Our results currently showed that Cx3cr1, Csf1r, Ms4a3 and P2ry12 may have the ability of tracing one or more synovial monocyte and macrophage populations. The gene and surface markers identified in each synovial monocyte, macrophage and TR-MC population will help develop better tools to trace and delete them. Future studies will also focus on determining the spatial topology of each synovial monocyte and macrophage population to better define their role in joint health.
To cite this abstract in AMA style:
Wang Y, Cuda C, Winter D, Perlman H. Fate-mapping of Synovial Monocytes and Macrophages [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/fate-mapping-of-synovial-monocytes-and-macrophages/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fate-mapping-of-synovial-monocytes-and-macrophages/