Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical I
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: 68Ga-FAPI-PET/CT is a novel imaging method monitoring in-vivo fibroblast activation. We recently described an association of 68Ga-FAPI-uptake with deterioration of Forced Vital capacity (FVC) after a six-month follow-up in a small cohort of SSc-ILD patients. The aim of the present study was to confirm the prognostic value of 68Ga-FAPI-PET/CT in a larger cohort over a longer follow-up-period. A novel threshold-based computational quantification approach was used.
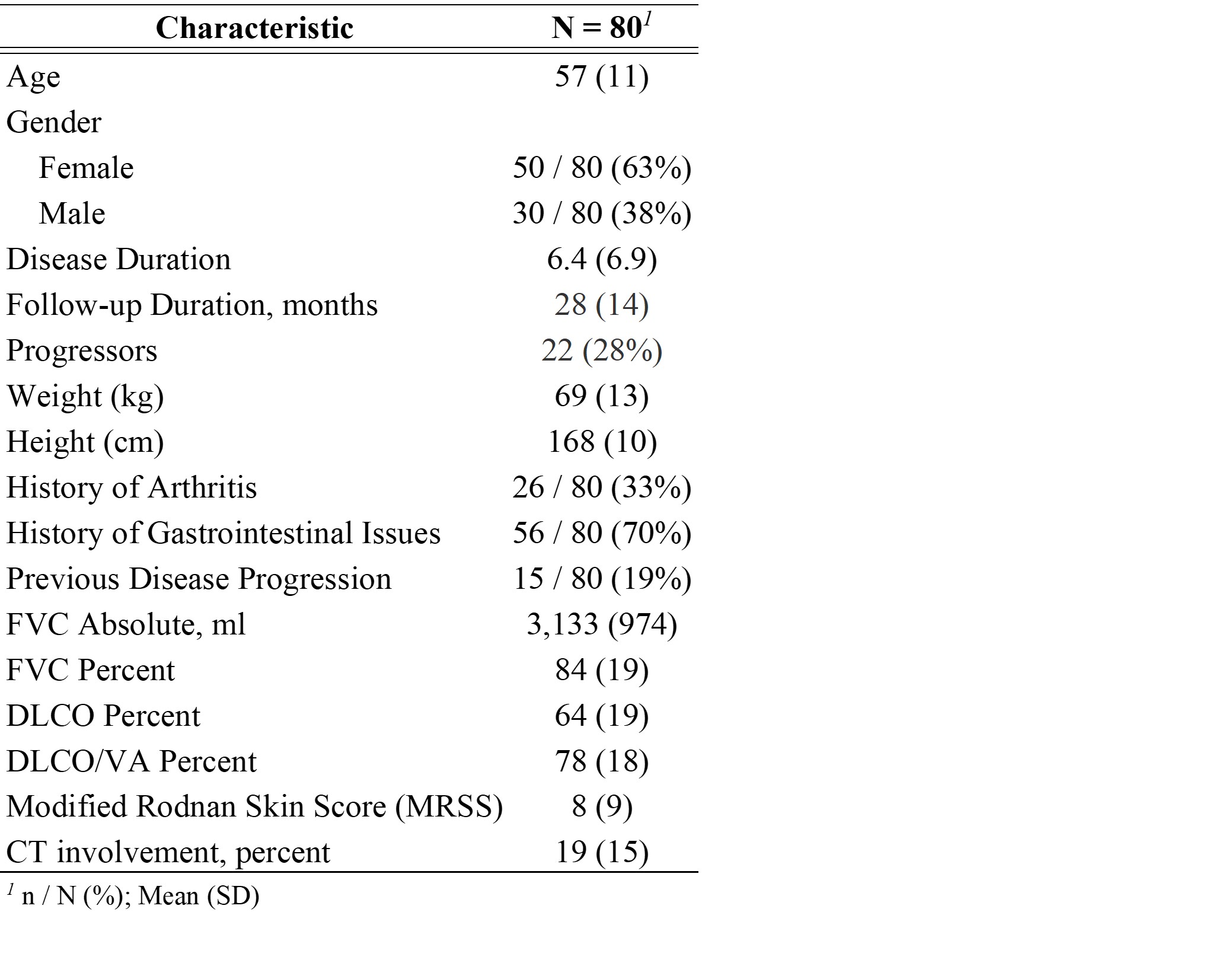
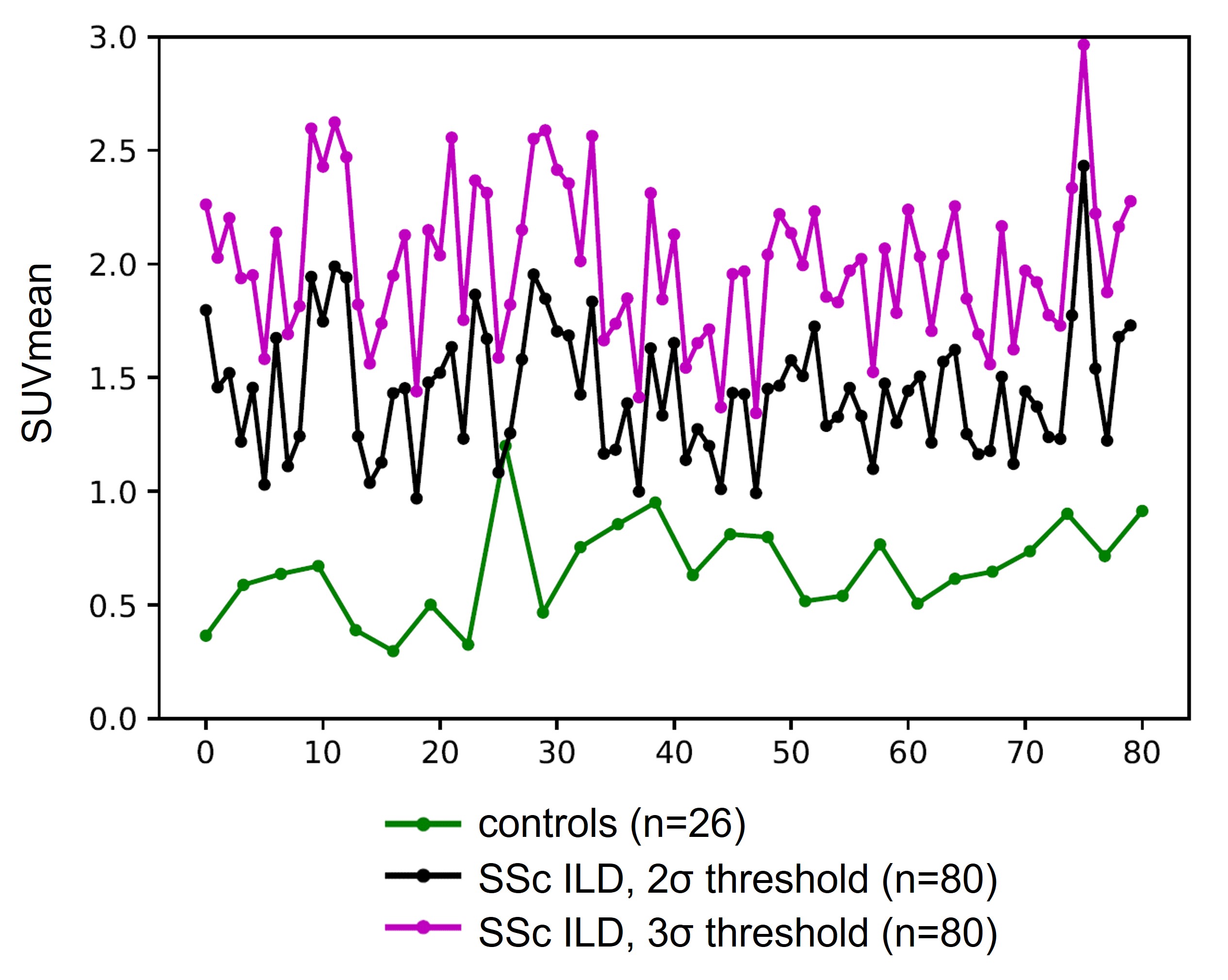
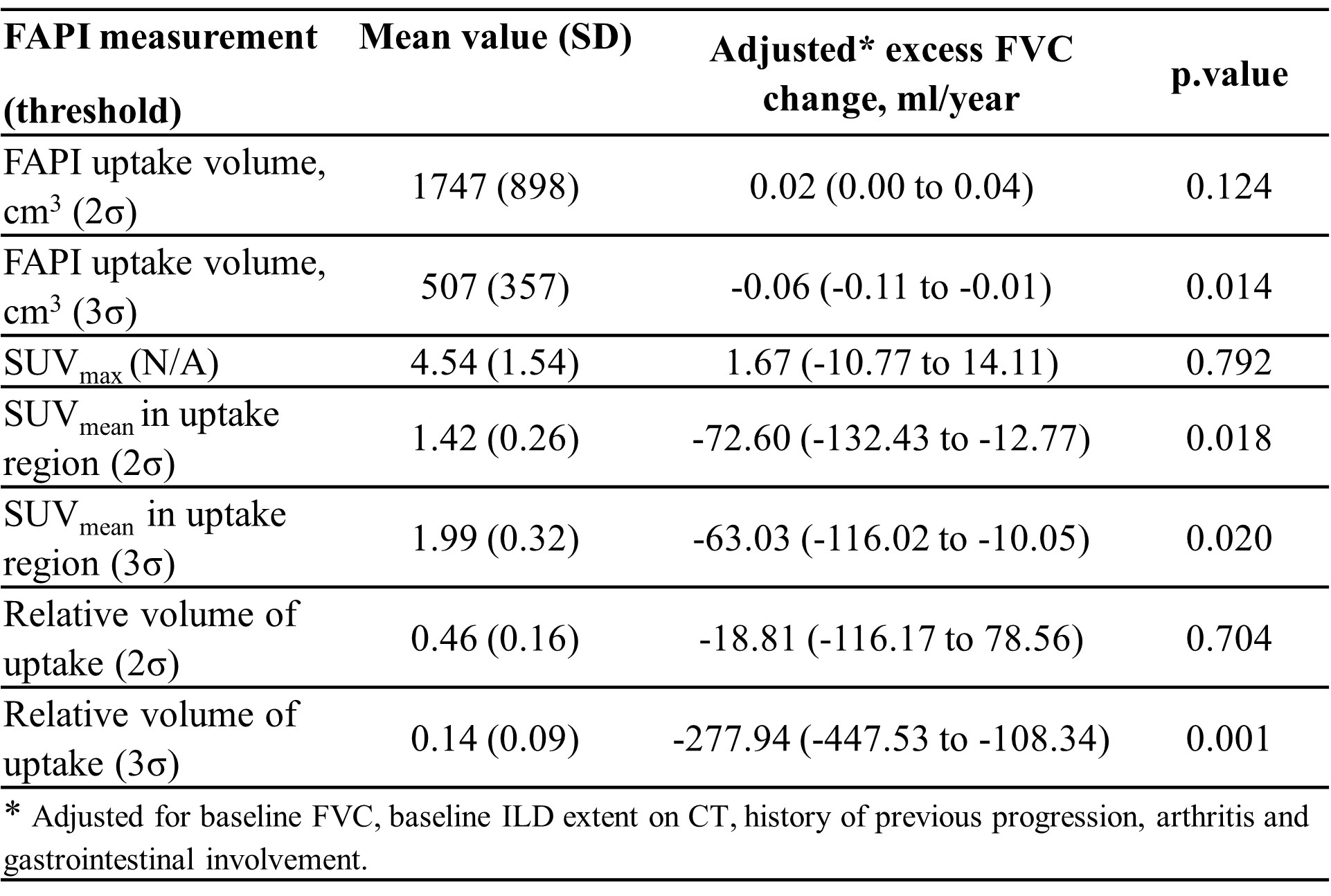
Methods: 80 SSc patients (ACR/EULAR criteria 2013), with HRCT-confirmed SSc-ILD and a baseline FAPI-PET scan were consecutively recruited at the University of Erlangen between 10/09/2018 and 22/02/2023 and serially followed with pulmonary function measurements. Yearly FVC-change (ml/year) calculated using linear regression was the primary outcome. FAPI-PET scans of 26 individuals without lung disease were used as reference to define background levels in non-diseased lungs. Abnormal FAPI uptake was defined as (I) 2σ (95% CI) and (II) 3σ (97,5%CI) above average signal in controls. Based on these, the volume (cm3) and intensity (SUV mean and max) of FAPI-uptake and relative FAPI-uptake volume were derived. We estimated an excess annual FVC loss (ml) associated with each unit of FAPI uptake increase for the different measures adjusted for baseline FVC and ILD-extent on CT scan, history of arthritis and gastrointestinal reflux disease (GERD) using mixed effects linear regression models.
Results: We included 80 patients with SSc-ILD, (age 57±11, 50 (63%) females). Baseline characteristics are summarized in table 1. 30 patients had diffuse SSc, 50 limited SSc. The different thresholds for FAPI uptake (SUV mean) tested resulted in separation of “Uptake” versus “Non-uptake lung” and threshold-based SUV mean values are visualized figure 1. Estimated median (IQR) FVC change was -6.0 (-34.8 to 21.8) ml/year (range, -196 to 187) and 22 patients (28%) were progressors based on the regression-projected 5-year FVC change exceeding 5% of baseline FVC. We evaluated the absolute volume of FAPI uptake, maximum uptake, average intensity in the uptake region and the proportion of lung volume showing FAPI uptake (table-2). Regardless of the threshold defining uptake, each unit increase in the average intensity of uptake was associated with an excess reduction in FVC of 63 to 72 ml per year after adjusting for progression risk factors already known. The absolute and relative volume of lung showing uptake was also significantly associated with an adjusted excess FVC reduction for the highest threshold level (table-2).
Conclusion: In this preliminary analysis we observed that higher FAPI intensity or relative uptake volume at baseline is associated with a greater rate of FVC decline after adjusting for described risk factors of SSc-ILD progression. The association with uptake volume was evident for the data obtained using the (3σ (97,5%CI) based threshold definition. These results indicate that the volume and intensity of FAPI uptake are indicators of accelerated lung function decline independent of the known risk factors for ILD progression.
To cite this abstract in AMA style:
Atzinger A, Huang Y, Tascilar K, Bayerl N, Distler J, Staudt K, Kartalczik H, Bruni C, Distler O, Hoffmann-Vold A, Bäuerle T, Prenner A, Putz F, Breininger K, Kuwert T, Kainz B, Schett G, Bergmann C. FAPI-PET/CT as a Predictor of Accelerated Lung Function Deterioration in Systemic Sclerosis Related Interstitial Lung Disease (ILD): Preliminary-interim Analysis from a Confirmatory Risk Factor Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/fapi-pet-ct-as-a-predictor-of-accelerated-lung-function-deterioration-in-systemic-sclerosis-related-interstitial-lung-disease-ild-preliminary-interim-analysis-from-a-confirmatory-risk-factor-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fapi-pet-ct-as-a-predictor-of-accelerated-lung-function-deterioration-in-systemic-sclerosis-related-interstitial-lung-disease-ild-preliminary-interim-analysis-from-a-confirmatory-risk-factor-study/