Session Information
Date: Tuesday, November 9, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III (1836–1861)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Systemic sclerosis (SSc) is a progressive autoimmune disease with high morbidity and mortality, making early diagnosis and management critical. Anti-Topoisomerase I antibody (anti-Topo I, also known as Scl-70) has important diagnostic and prognostic roles in SSc. Although positivity for anti-Topo I antibody is considered highly specific for SSc, the testing methods typically utilized in commercial laboratories have lower specificity than the immunodiffusion method. To highlight potential harms of a false-positive anti-Topo I antibody result, we describe a series of cases in which patients who tested positive for anti-Topo I antibody in a commercial laboratory were found to be negative for anti-Topo I antibody by the immunodiffusion method.
Methods: Patients seen in our clinic who had previously tested positive for anti-Topo I antibody performed by a commercial laboratory, but who lacked sufficient clinical criteria to be classified as SSc, were invited to undergo testing for anti-Topo I antibody by immunodiffusion in our research laboratory. Each patient’s clinical features, commercial anti-Topo I antibody results, additional diagnostic testing, and clinical diagnosis at initial and follow up visits were recorded.
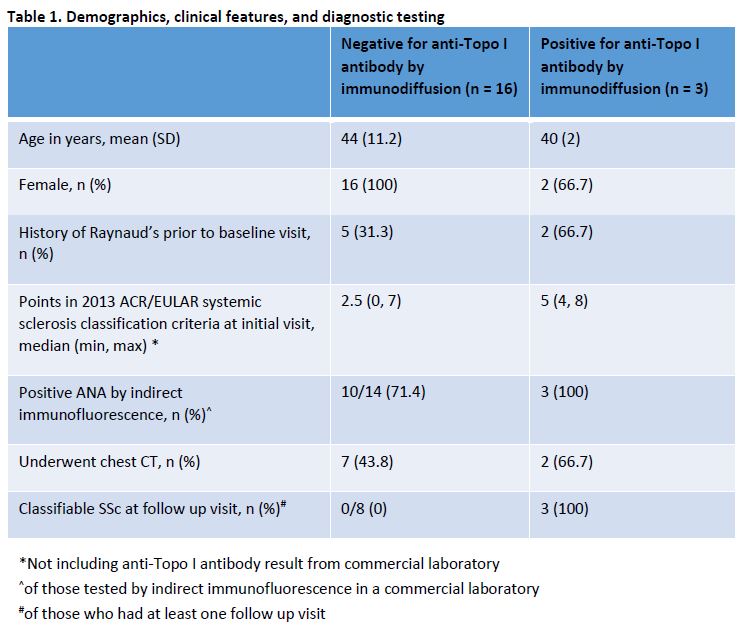
Results: Over a six-year period, 19 patients who fulfilled the above criteria underwent testing for anti-Topo I antibody by immunodiffusion (Table 1). 16 (84.2%) were found to be negative. 11 of the 19 patients (57.9%) had at least one follow up visit, typically 6-12 months after initial visit. Of those 11 patients with at least one follow up visit, all three patients who tested positive for anti-Topo I antibody by immunodiffusion, but none of the eight patients who tested negative, developed sufficient criteria to be classified as SSc on follow up. One of the patients with a false-positive anti-Topo I antibody result was diagnosed with morphea, one with Sjogren’s syndrome, one with antiphospholipid antibody syndrome, and two with undifferentiated connective tissue disease. The other 11 patients with false-positive anti-Topo I antibody results (68.8%) did not have a rheumatologic condition in the judgement of the treating physician. 7 of the 16 patients with false-positive anti-Topo I antibody (43.8%) had undergone chest CTs to screen for interstitial lung disease, one had been started on treatment with Mycophenolate Mofetil, and one had undergone pre-evaluation for autologous hematopoietic stem cell transplantation.
Conclusion: Our findings highlight the propensity of commercial laboratories to produce false-positive anti-Topo I antibody results. In the cases reported here, unnecessary referrals to a scleroderma specialty clinic, and in some cases unnecessary chest CTs, occurred after patients tested falsely positive for anti-Topo I antibody, demonstrating some of the potential negative impacts on patients and the healthcare system. While a thorough history and physical exam remain the mainstays of SSc evaluation, testing for anti-Topo I antibody using a more specific method such as immunodiffusion would likely help physicians and patients to avoid misdiagnoses, unnecessary referrals and diagnostic testing, and potentially risky treatments.
To cite this abstract in AMA style:
Lam B, Taherian R, Charles J, Mayes M, Assassi S, Skaug B. False Positive Anti-Topoisomerase I (Scl-70) Antibody Results: A Case Series from a Scleroderma Referral Center [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/false-positive-anti-topoisomerase-i-scl-70-antibody-results-a-case-series-from-a-scleroderma-referral-center/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/false-positive-anti-topoisomerase-i-scl-70-antibody-results-a-case-series-from-a-scleroderma-referral-center/