Session Information
Date: Monday, November 13, 2023
Title: (1345–1364) Reproductive Issues in Rheumatic Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Pregnancies in women with chronic diseases are often accompanied by concerns about potential complications [1]. This analysis explored medication-related concerns among women with inflammatory rheumatic disease (IRD) and identified influencing factors.
[1] PMID: 26039501
Methods: The German registry Rhekiss is a nationwide, multicentre, web-based cohort study to investigate pregnancies in women with various IRD. Women can participate if they are either planning a pregnancy (cohort 1) or are already pregnant (up to 20 weeks of gestation; cohort 2). Rheumatologist- and patient-reported data are captured at regular, pre-defined follow-up visits.
We analysed data of women who had responded to a question about concerns regarding pregnancy and child’s health in relation to medication. Categorical answers and patient characteristics were analysed descriptively. The impact of factors associated with patients’ anxieties was estimated by multivariable ordinal logistic regression using the proportional odds model and combining the two cohorts. Missing values were replaced by single imputation.
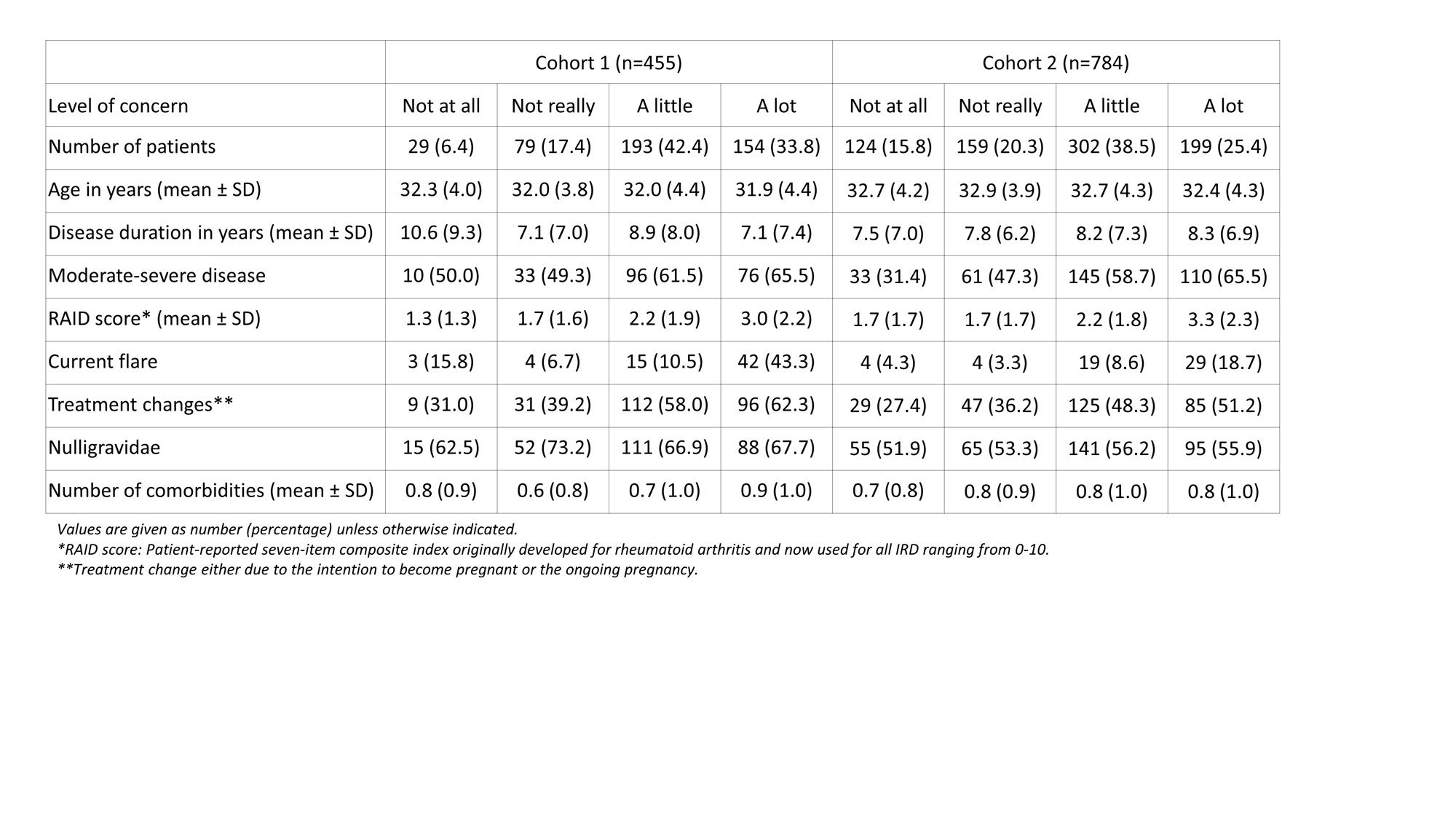
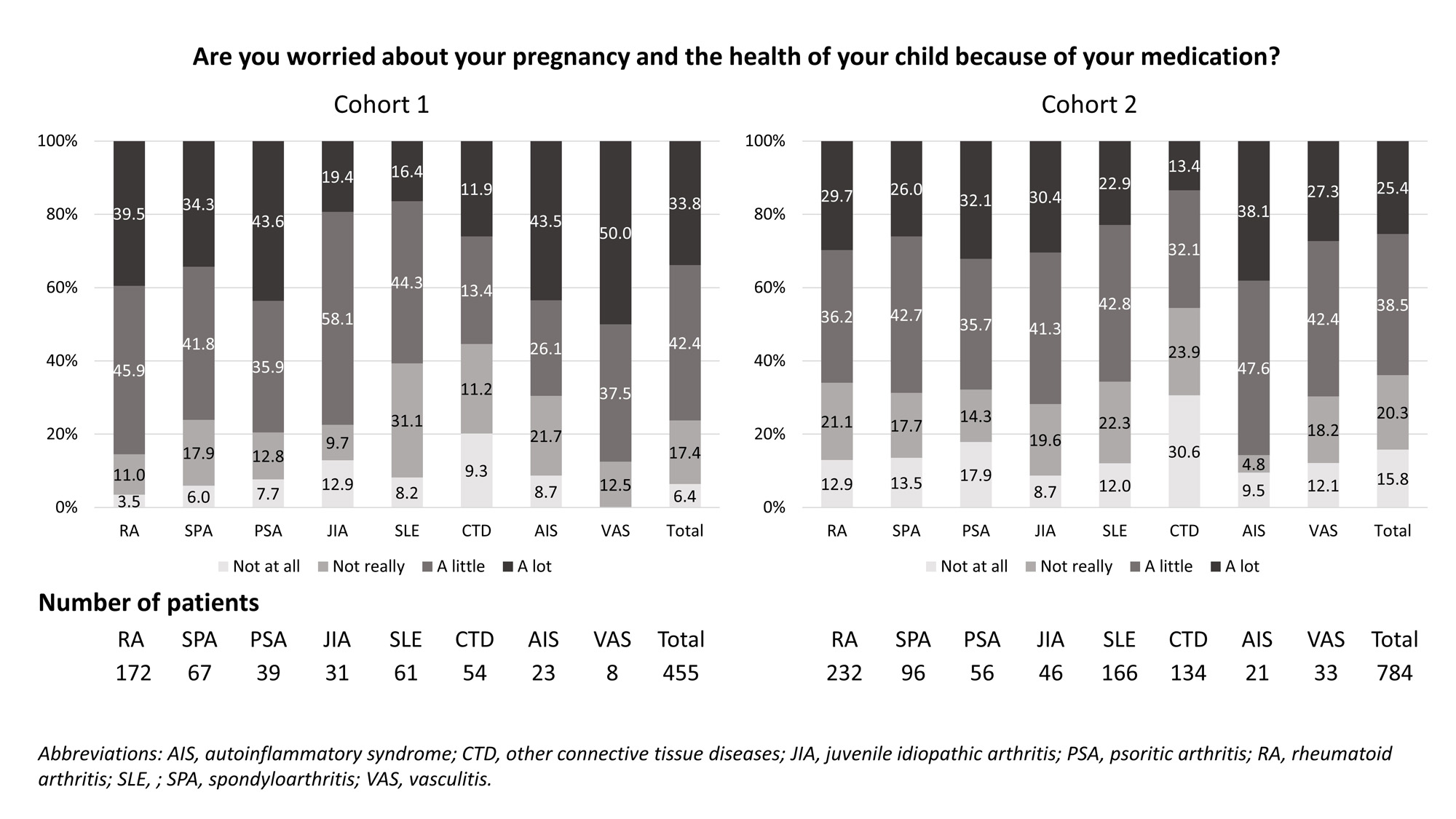
Results: Between 09/2015 and 10/2022, a total of 2,240 patients were enrolled (cohort 1: 708, cohort 2: 1,532). Out of those, n=455 and n=784, respectively, were eligible for the analysis.On average, women were 32-33 years old and disease duration was 7-11 years (table).A total of 34% in cohort 1 and 25% in cohort 2 stated that they are concerned “a lot” (figure). Responses varied across different IRD diagnoses.
Treatment changes had the greatest impact on higher levels of worries, (odds ratio 1.72 [95% confidence interval 1.38; 2.14]). Furthermore, a significant association was found for moderate-severe IRD (1.34 [1.17; 1.53]) and a higher disease impact, measured by patient-reported RAID score (per 1-point-increase: 1.31 [1.24; 1.38]). Patients who were already pregnant at enrolment were less worried than patients planning a pregnancy (0.63 [0.51; 0.79]). No significant relationship was found for maternal age, number of prior pregnancies and comorbidities.
Conclusion: At least one in four women with IRD is “very concerned” about pregnancy and infant health due to medications. Treatment changes, moderate-severe disease and higher RAID score were associated with higher levels of concern. Therefore, worries should be an essential part of individual counselling for patients who either wish to become pregnant or are already pregnant.
Funding: Rhekiss is jointly funded by the German Rheumatism Research Centre Berlin and the Rheumazentrum Rhein-Ruhr e.V., Düsseldorf.
To cite this abstract in AMA style:
Meissner Y, Eickhoff B, Glaser C, Henes J, Richter J, Spaethling-Mestekemper S, Specker C, Fischer-Betz R, Strangfeld A. Factors Associated with Medication-related Concerns in Women with Inflammatory Rheumatic Diseases – an Analysis of a Nationwide Pregnancy Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/factors-associated-with-medication-related-concerns-in-women-with-inflammatory-rheumatic-diseases-an-analysis-of-a-nationwide-pregnancy-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-medication-related-concerns-in-women-with-inflammatory-rheumatic-diseases-an-analysis-of-a-nationwide-pregnancy-cohort/