Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: The Symptom Severity Index (SSI) is a patient-reported outcome measure (PROM) developed for IgG4-related disease (IgG4-RD). The SSI assesses symptoms of IgG4-RD and the distress they cause. It queries patients on symptoms due to active disease as well as those that might be a result of organ damage. The face, content, and construct validity were previously established. However, remaining aspects of validation remained incomplete. Here, we report test-retest reliability of the SSI, responsiveness to treatment, and construct validity with damage.
Methods: The SSI assesses 24 symptoms across 9 categories which correspond with clinical manifestation. Participants report symptoms experienced in the prior 30 days; only symptoms associated with IgG4-RD organ involvement known to be present in the participant are counted. For each symptom, a symptom distress score is calculated by multiplying symptom frequency (range “Never” [0 points] to “Every Day” [3 points]) by associated distress (range “None” [0 points] to “Very Much” [4 points]). Each symptom distress score is summed to determine a total SSI score (range: 0 to 288). We administered the SSI to consecutive patients who fulfilled the ACR/EULAR Classification Criteria in a single center. To assess test-retest reliability, we administered the SSI twice to participants within 7 days and assessed the Pearson correlation. We assessed responsiveness to treatment of the total SSI score in participants who completed the SSI prior to and following treatment initiation during a time when response to treatment was expected using a paired t test. Construct validity with damage was assessed by measuring the correlation between the SSI total score and the number of sites with damage from IgG4-RD.
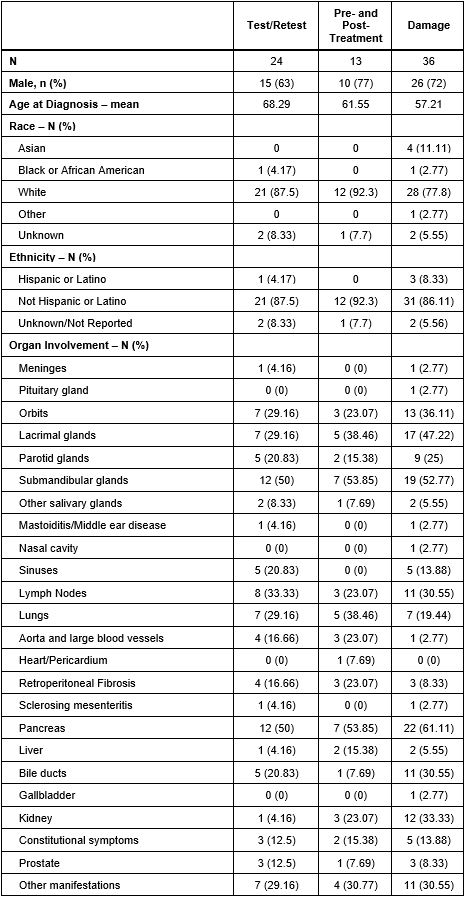
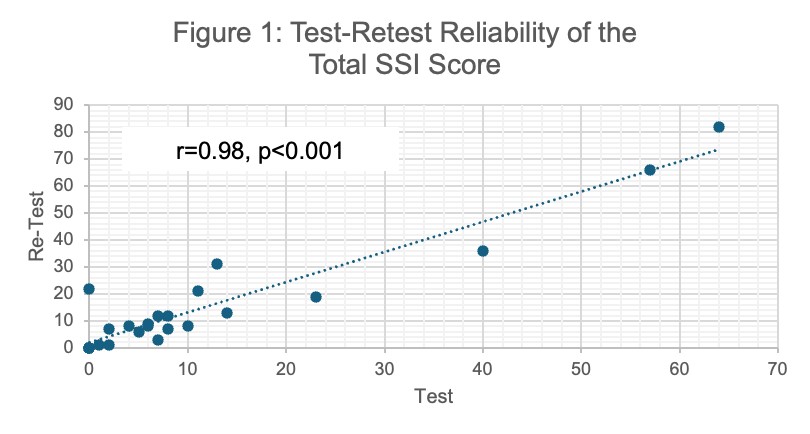
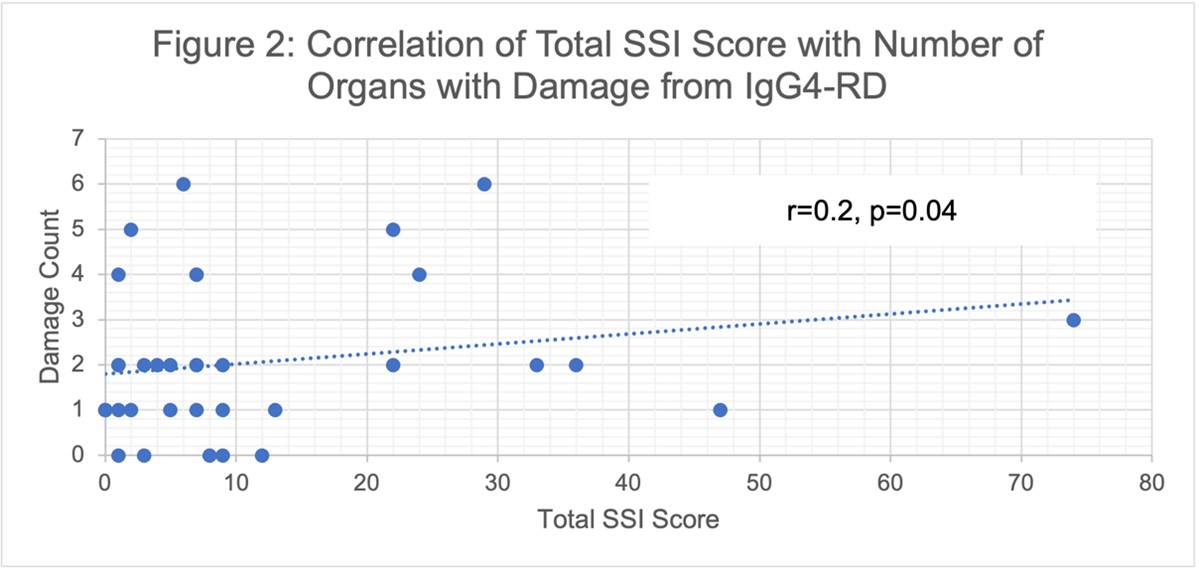
Results: The demographics of participants whose data contributed to each step of this validation exercise are reported in Table 1. 24 patients completed the SSI twice (mean [SD] days between surveys 3.1 [1.4]). The mean (SD) total SSI score on the first and second assessments were 12 (17.4) and 15.5 (20.6), respectively. We found strong test-retest reliability with a correlation coefficient of 0.98 (Figure 1, p< 0.001). 13 patients completed the SSI prior to and after treatment (mean (SD) days between surveys 205 [64]); 9 received B cell depletion and 4 received prednisone. The mean total SSI score decreased from 27.2 (30.1) to 11.1 (11.2) (difference 16.1 [95%CI 0.75 to 31.41] p=0.04) before and after treatment, respectively. 36 patients completed the SSI and had an assessment of damage at the time of SSI; of these, the median number of sites of damage was 2 (IQR 1, 2.75). The SSI total score correlated with the number of sites of damage by IgG4-RD (Figure 2, correlation coefficient of 0.21 [p=0.04]).
Conclusion: The IgG4-RD SSI is a disease-specific PROM with strong validity. We demonstrate that in addition to face, content, and construct validity with quality of life and disease activity, the SSI has good test-retest reliability, responsiveness to treatment, and construct validity with damage. The SSI is currently in use as part of an ongoing randomized controlled trial in IgG4-RD.
To cite this abstract in AMA style:
Jha I, McMahon G, Katz G, Srivatsan S, Bolden C, Fernandes A, Perugino C, Stone J, Wallace Z. Extended Validation of the IgG4-Related Disease Symptom Severity Index (SSI) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/extended-validation-of-the-igg4-related-disease-symptom-severity-index-ssi/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/extended-validation-of-the-igg4-related-disease-symptom-severity-index-ssi/