Session Information
Session Type: Abstract Session
Session Time: 11:00AM-11:50AM
Background/Purpose: Rituximab is an effective therapy for induction of remission in ANCA-associated vasculitis (AAV). However, the effect of rituximab is not sustained, and subsequent relapse rates are high, especially in patients with a history of relapse. The RITAZAREM trial (ClinicalTrials.gov identifier: NCT01697267) was an international, multi-center, open-labelled, randomized, controlled trial of patients with AAV with relapsing disease comparing the efficacy, after induction of remission with rituximab, of two relapse-prevention strategies: repeat dosing of rituximab or daily oral azathioprine. The extended follow-up phase of the trial provides a unique opportunity to ask important questions about durability of effects of therapies, safety of extended courses of azathioprine and rituximab, and clinical and laboratory biomarkers of remission and relapse in AAV.
Methods: Patients with AAV were recruited at the time of relapse and received induction therapy with rituximab and glucocorticoids. If remission was achieved by month 4, patients were randomized in a 1:1 ratio to receive either rituximab (1000 mg every 4 months for 5 doses) or azathioprine (2 mg/kg/day) as maintenance therapy. Patients were followed for a minimum of 36 months, with most patients followed for 48 months. The primary outcome of the trial was time to disease relapse. The trial was completed in November 2019.
Results: 188 patients were enrolled and 170 randomized at 4 months (85 to rituximab; 85 to azathioprine). Median age was 59 years (range 19-89), with a prior disease duration of 5.3 years (0.4-38.5). 123/170 (72%) patients had a history of testing positive for anti-proteinase 3-ANCA; 47/170 (28%) for myeloperoxidase-ANCA. 104/170 (61%) were enrolled having suffered a major relapse, and 48/170 (28%) received a pre-specified higher dose glucocorticoid induction regimen (Table 1). 114 (67%) patients had prior renal involvement. Previously presented data demonstrated the superiority of rituximab over azathioprine during the maintenance treatment period. Results of the follow-up phase of the study after discontinuation of maintenance therapy will be presented at the meeting and include analyses of clinical courses and the utility of biomarkers to predict relapse and/or sustained remission, including ANCA titers, B cell counts, and immunoglobin levels.
Conclusion: The results of RITAZAREM through month 48 will demonstrate whether rituximab leads to sustained remission beyond the treatment period in patients with AAV with a prior history of relapse following induction of remission with rituximab. These data will also inform clinicians and patients about the longer-term safety of azathioprine and rituximab and provide important insight into the pathophysiology of relapse and remission in AAV.
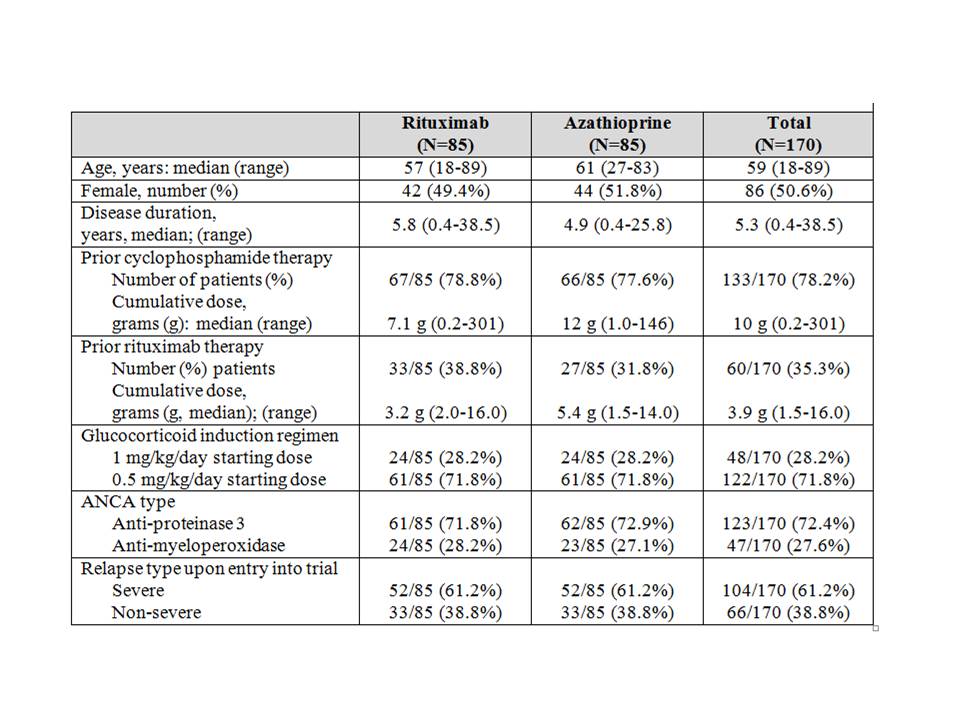 Table 1: Baseline characteristics of patients enrolled in RITAZAREM trial
Table 1: Baseline characteristics of patients enrolled in RITAZAREM trial
To cite this abstract in AMA style:
Smith R, Jayne D, Merkel P. Extended Follow-Up of Patients Recruited to a Randomized, Controlled Trial of Rituximab versus Azathioprine After Induction of Remission with Rituximab for Patients with ANCA-Associated Vasculitis and Relapsing Disease [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/extended-follow-up-of-patients-recruited-to-a-randomized-controlled-trial-of-rituximab-versus-azathioprine-after-induction-of-remission-with-rituximab-for-patients-with-anca-associated-vasculitis-and/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/extended-follow-up-of-patients-recruited-to-a-randomized-controlled-trial-of-rituximab-versus-azathioprine-after-induction-of-remission-with-rituximab-for-patients-with-anca-associated-vasculitis-and/
