Session Information
Date: Monday, November 18, 2024
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
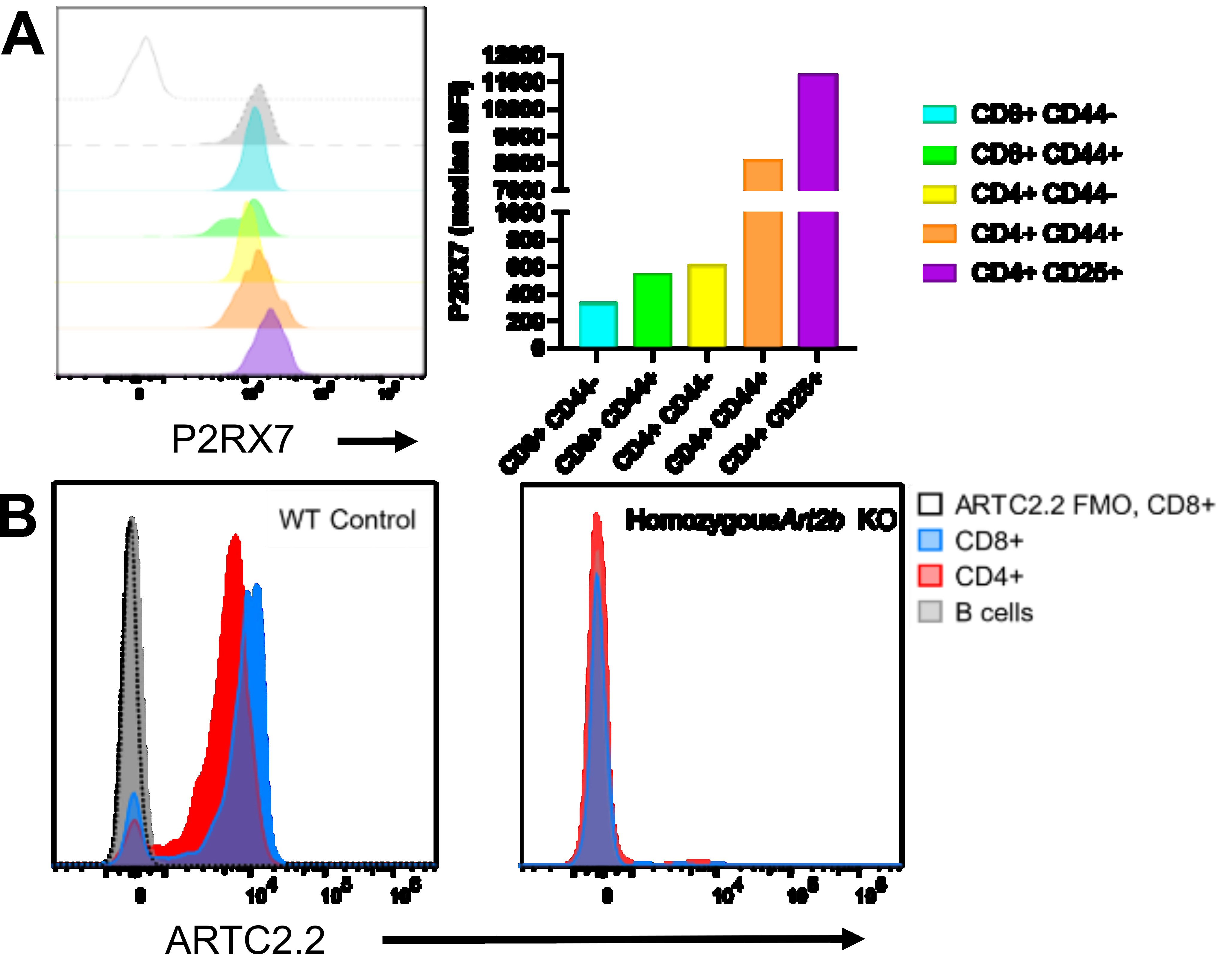
Background/Purpose: Extracellular NAD+ and ATP are two of the many damage-associated molecular pattern molecules (DAMPs) released at sites of tissue damage which modify activities of the innate and adaptive immune systems. Among cell-surface proteins which detect these molecules in mice, the NAD+-responsive, ADP-ribosyltransferase ARTC2.2 and the ATP-dependent ion channel P2RX7 represent two members of a pathway which controls T cell homeostasis. P2RX7 is pivotal for development and maintenance of long-lived central and tissue-resident memory T cells (TRM). However, covalent ADP-ribosylation of P2RX7 by ARTC2.2 in response to NAD+ enhances persistent non-selective pore formation by P2RX7, causing activation of matrix metalloproteases which cleave cell surface markers including CD27 and CD62L, cell membrane depolarization, and cell death. ARTC2.2/P2RX7 activity occurs at both physiologic levels of NAD+ and ATP during the extraction of immune cells from tissues for investigation, leading to cell death. Amongst cells with high expression of both ARTC2.2 and P2RX7, this sensitivity to so-called NAD-induced cell death (NICD) has led to methodologic difficulties extending characterization of immune responses from murine models to human physiology. Furthermore, humans lack a functional equivalent of ARTC2.2.
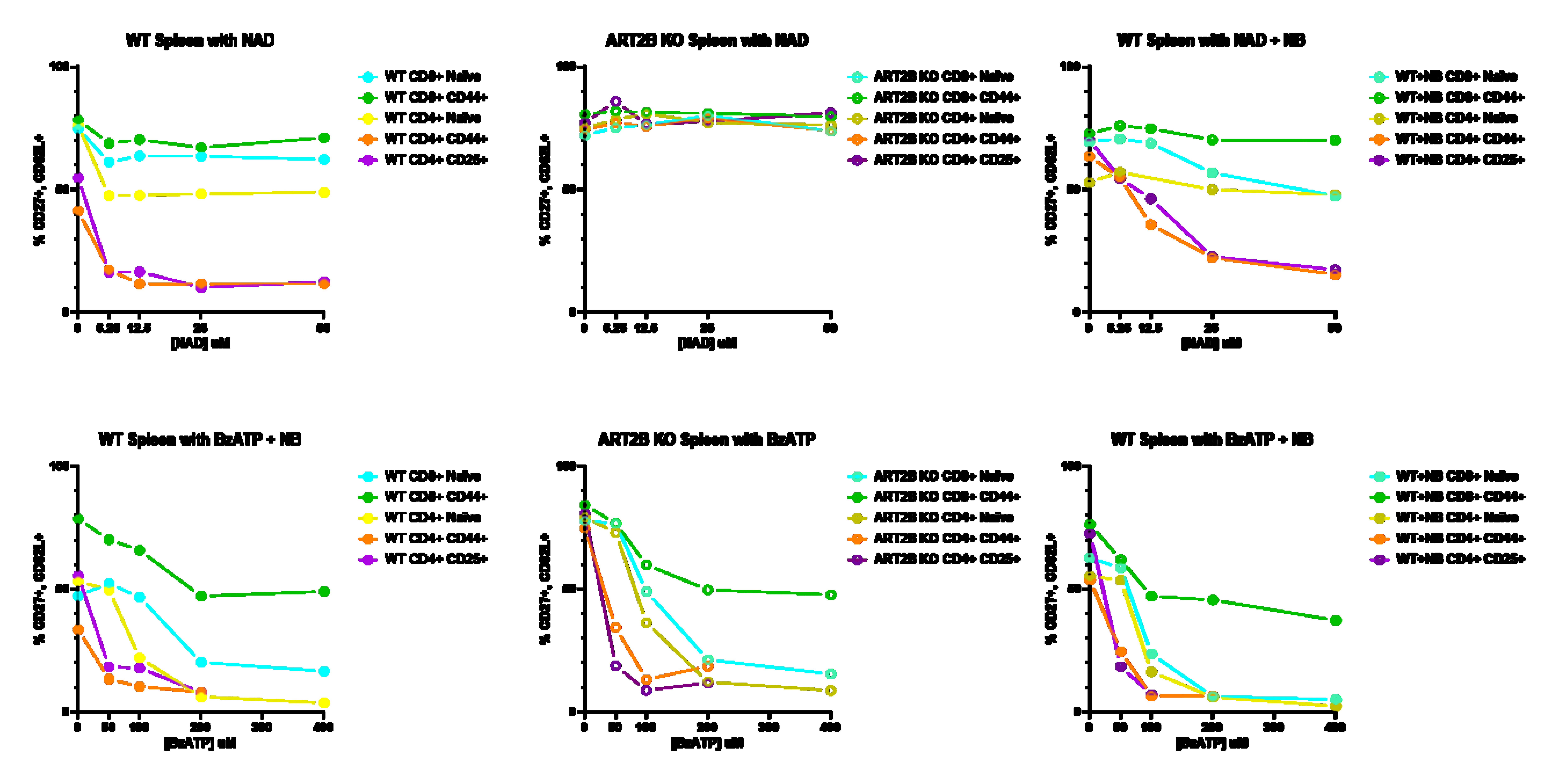
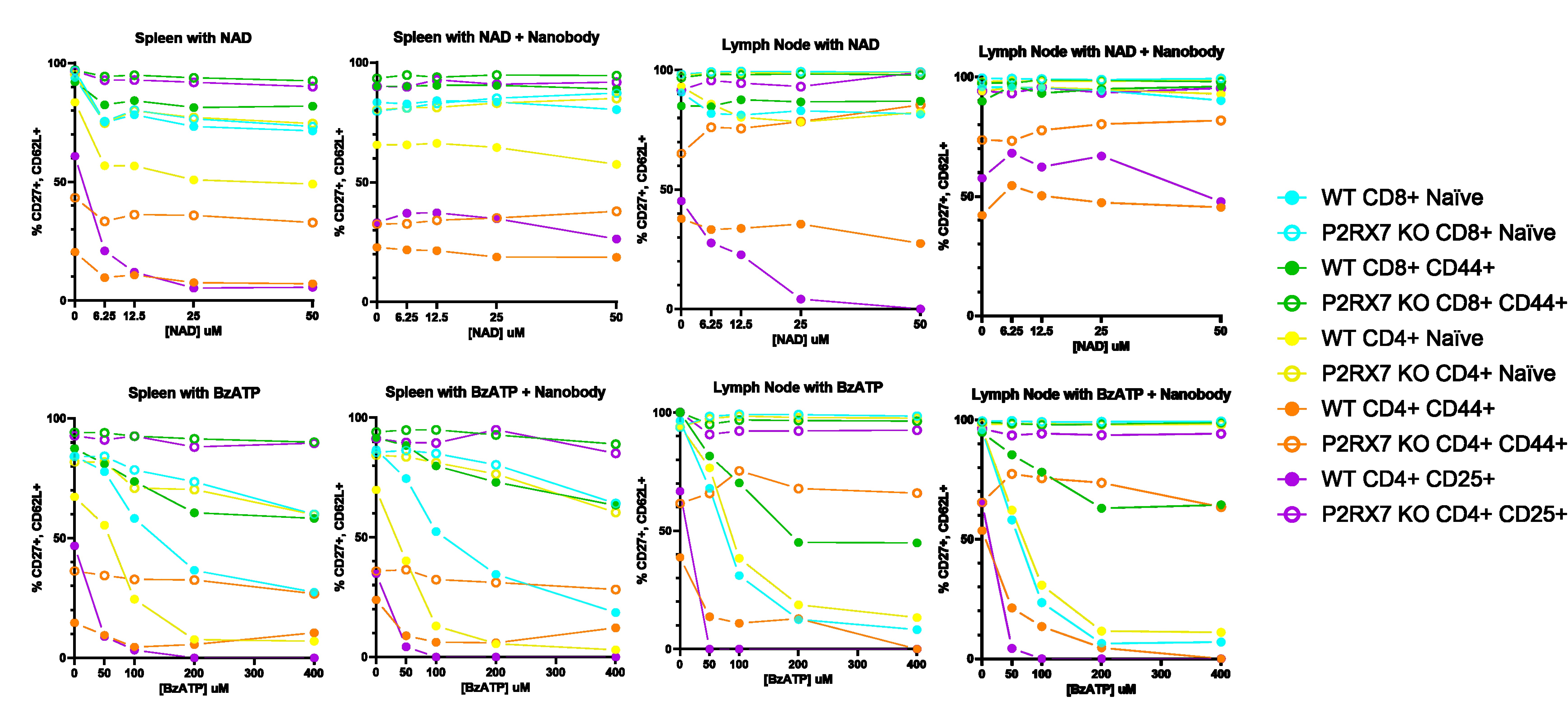
Methods: We developed a novel, germline Art2b -/- mouse line via CRISPR. Circulating, lymph node-derived, splenic, or tissue-resident lymphocytes from Art2b -/- mice, P2rx7 -/- mice, and wild-type controls were extracted conventionally and/or in the presence of ARCT2.2 antagonistic nanobody or commercially-available P2RX7-specific small-molecule inhibitor A-740003. Lymphocytes were subjected ex vivo to incubation with NAD+ or ATP analogue, bzATP. Mixed tissue isolates were assessed by spectral flow cytometry for viability and presence of cell surface markers.
Results: Art2b -/- mice resist ex vivo NICD compared to WT controls across all major conventional T-cell subsets tested. Consistent with known ARTC2.2 function, downstream activation of P2RX7 and proteolytic cleavage of CD27 and CD62L following NAD exposure is near-completely obviated in Art2b -/- T cells, whereas sensitivity to extracellular ATP is preserved. P2rx7 -/- mice or treatment with A-740003 attenuate, NICD, ATP-induced cell death, and CD27/CD62L shedding. Surprisingly, naïve CD8+ T cells demonstrate particular P2RX7-mediated sensitivity to extracellular ATP, despite minimal expression of P2RX7. Additionally, Art2b -/- mice have greater retrieval of viable T cell populations compared to WT or ARTC2.2 antagonistic nanobody.
Conclusion: Our results with naïve CD8+ T cells suggest a greater role for ARTC2.2/P2RX7 pathway activity in regulating T cell phenotype and homeostasis than is conventionally assumed. These investigations provide a promising inroad to further characterize the roles of NAD- and ATP-induced cell death on both naïve and mature T cell populations and the full diversity of tissue-resident T cells as they relate to models of acute and chronic diseases. As well, since ARTC2.2-mediated NICD is unique to rodents, further studies using Art2b-/- mice may provide a more accurate model for regulation human T cells.
To cite this abstract in AMA style:
Valente W, Rhee K, Wanhainen K, Jameson S. Expression Levels of the ATP-Gated Ion Channel P2RX7 Do Not Predict T Cell Sensitivity to Extracellular ATP and NAD [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/expression-levels-of-the-atp-gated-ion-channel-p2rx7-do-not-predict-t-cell-sensitivity-to-extracellular-atp-and-nad/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/expression-levels-of-the-atp-gated-ion-channel-p2rx7-do-not-predict-t-cell-sensitivity-to-extracellular-atp-and-nad/