Session Information
Date: Sunday, November 13, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster II: Manifestations
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: The goal of this exploratory study is to determine if urine:serum fractional excretion ratios can outperform the corresponding urinary biomarker proteins in identifying active renal disease in systemic lupus erythematosus (SLE).
Methods: Ninety-six adult SLE patients and twenty-five healthy controls were examined for serum and urine levels of 8 protein markers, namely ALCAM, Calpastatin, hemopexin, Peroxiredoxin 6 (PRX6), Platelet factor 4 (PF4), properdin, TFPI and VCAM-1, by ELISA. Fractional excretion of analyzed biomarkers was calculated after normalizing both the urine and serum biomarker levels against creatinine using the following equation, the same way fractional excretion of sodium is calculated: Fractional Excretion (FE)= (Urine biomarker/urine Cr) ÷ (Serum biomarker/serum Cr).
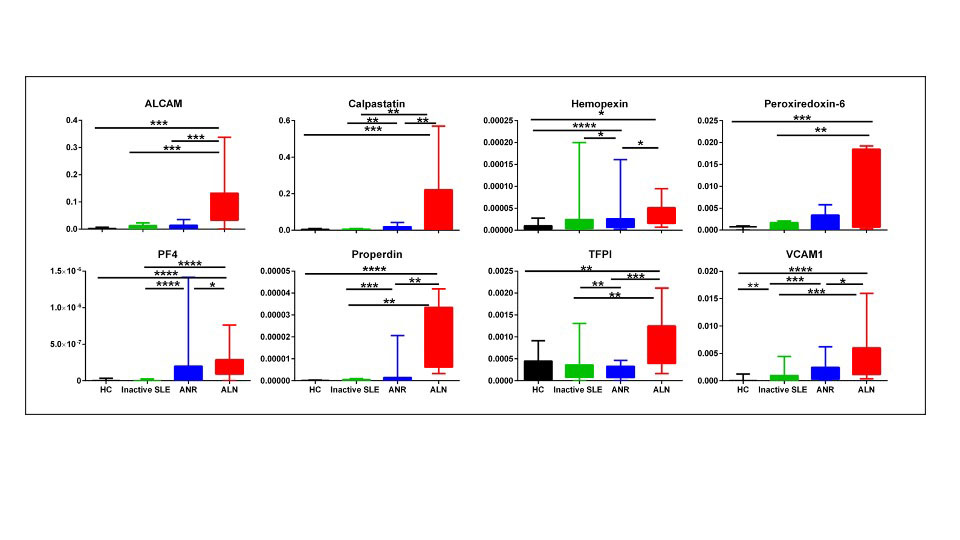
SLE disease activity was assessed using SLEDAI-2K. Renal activity was assessed using the renal domain scores of SLEDAI (range 0–16; 0 = inactive LN). At enrollment, patients were categorized into 3 groups; active renal SLE (LN, patients with renal SLEDAI score ≥ 4), active non-renal SLE (patients with total clinical SLEDAI ≥1, but renal SLEDAI=0) and inactive SLE (patients with total clinical SLEDAI = 0, asymptomatic with no findings of organ activity, subclinical hypocomplementemia and/or elevated autoantibodies allowed). The active renal group’s renal histopathologic features were analyzed using a kidney biopsy.
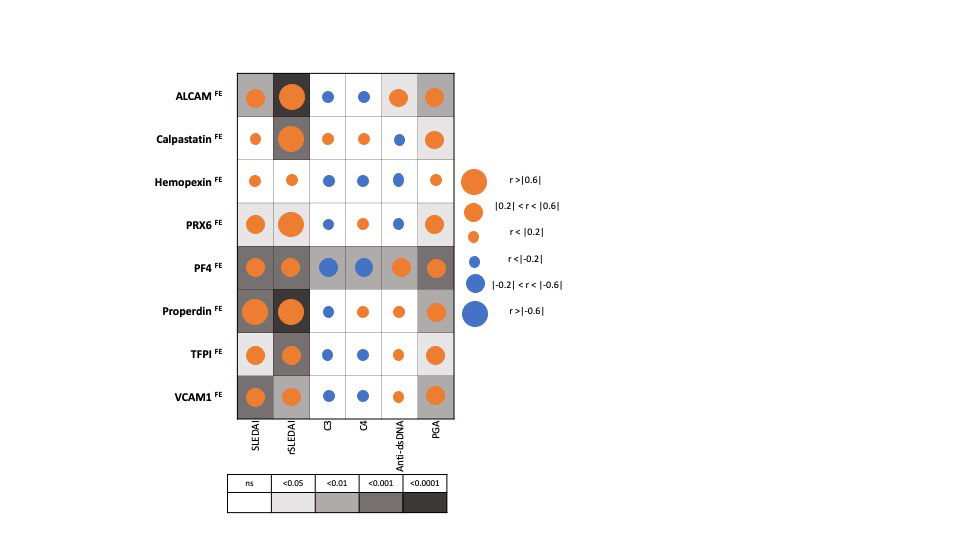
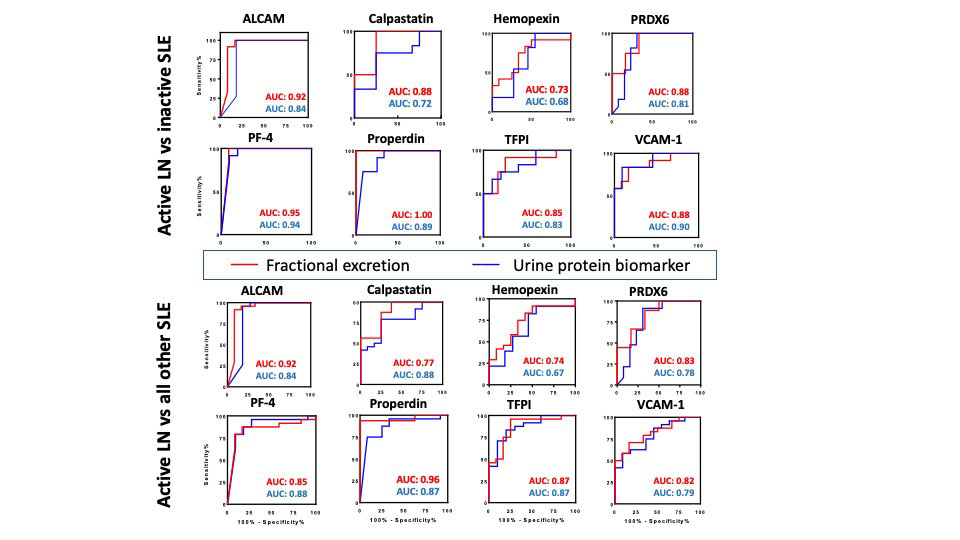
Results: The FE ratios of all 8 proteins interrogated outperformed conventional disease activity markers such as anti-dsDNA, C3 and C4 in identifying renal disease activity. All FE values were superior to the corresponding urine biomarkers levels in differentiating LN activity. Urine levels of all biomarker proteins assayed were highest among active LN patients, however, the serum levels of the assayed proteins from the same patients were not significantly different between the SLE patient groups among levels of sALCAM, sHPX, sPRX6 and sProperdin. PF4FE correlated significantly with all conventional disease activity parameters including SLEDAI, renal SLEDAI, PGA, complement C3, C4 and anti-dsDNA. Renal SLEDAI scores exhibited the best correlation coefficients with FE ratios of the assayed biomarkers, showing strong significant correlation with properdinFE (Rho 0.79, P< 0.0001), followed by ALCAMFE (Rho 0.64, P< 0.0001), CalpastatinFE(Rho 0.64, P< 0.001), and PRX6FE (Rho 0.64, P 0.01). PF4FE, TFPIFE and VCAM-1FE values showed “good” significant correlations with renal SLEDAI (Rho 0.58, 0.58, 0.49 respectively). ALCAMFE, PF4FE and properdinFE ratios exhibited the highest accuracy (AUC >0.9) in distinguishing active LN from inactive SLE. Four of the FE ratios exhibited perfect sensitivity (Calpastatin, PRDX6, PF4 and properdin), while ALCAMFE, PF4FE and properdinFE exhibited the highest specificity values for active LN.
Conclusion: With most of the tested proteins, the urine:serum fractional excretion ratios outperformed corresponding urine protein measurements in identifying active renal involvement in SLE. Hence, this novel class of biomarkers in SLE ought to be systemically evaluated in larger independent cohorts for their diagnostic utility in LN assessment.
To cite this abstract in AMA style:
Soliman S, Stanley S, Vanarsa K, Ismail F, Mok C, mohan C. Exploring the Potential of Urine: Serum Fractional Excretion Ratios as Disease Biomarkers in Active Lupus Nephritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/exploring-the-potential-of-urine-serum-fractional-excretion-ratios-as-disease-biomarkers-in-active-lupus-nephritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-the-potential-of-urine-serum-fractional-excretion-ratios-as-disease-biomarkers-in-active-lupus-nephritis/