Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) have transformed cancer treatment, offering significant benefits in various malignancies. However, these therapies can induce immune-related adverse events (irAEs), including polymyalgia rheumatica (PMR), giant cell arteritis (GCA), and anterior ischemic optic neuropathy (AAION). Understanding the differential risks is crucial for optimizing patient management. This retrospective database analysis compares the outcomes between patients treated with pembrolizumab and those receiving the combination therapy of nivolumab and ipilimumab. Using the TrinetX database, which gathers electronic health records from 88 healthcare organizations worldwide, our study aims to uncover important insights into the autoimmune safety profiles of these immunotherapies.
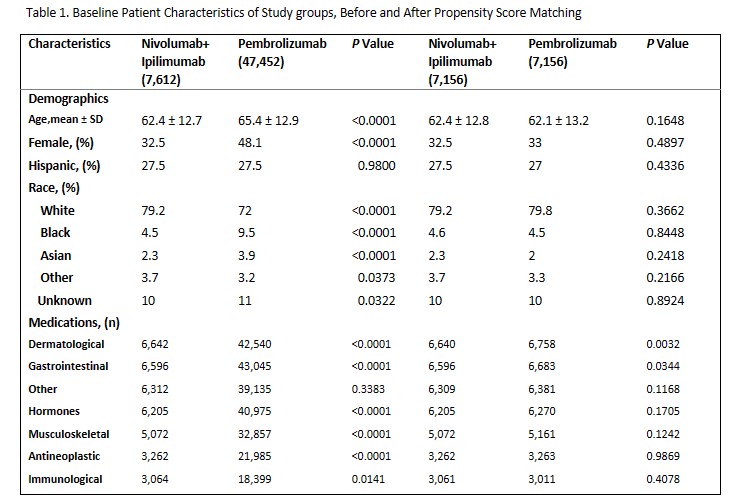
Methods: In this study, patients aged 18-99 were assigned to either the nivolumab and ipilimumab group or the pembrolizumab group. Inclusion criteria encompassed various cancer types (Appendix 1). Exclusion criteria involved the use of alternative ICIs. Diagnoses were identified via ICD-10 codes and medications were identified by RxNorm codes. 1:1 propensity score matching was performed for age, gender, ethnicity, medication use (Table 1). The index event comprised the appearance of oncological diagnosis and ICIs in patient records. The primary outcomes included the diagnosis of PMR, GCA, and AAION following ICI initiation, patients with pre-existing conditions were excluded from analysis.
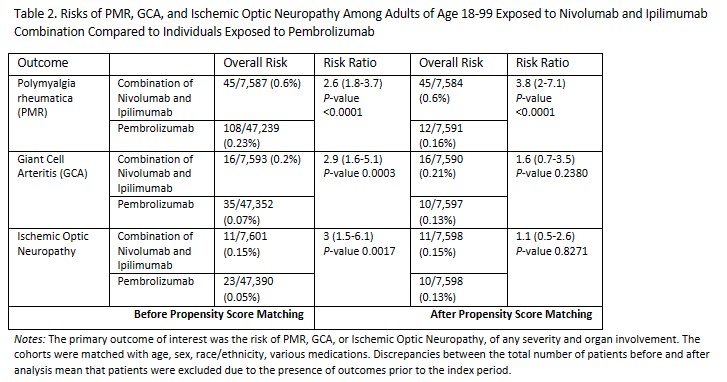
Results: Following the initial analysis, the combination group comprised 7,612 individuals receiving nivolumab and ipilimumab, while the pembrolizumab group consisted of 47,452 patients. The nivolumab and ipilimumab combination group tended to be slightly younger, the pembrolizumab group exhibited a more uniform gender distribution, with around 70% males in the nivolumab and ipilimumab combination group. Both cohorts were predominantly composed of white patients. Before propensity score matching, the nivolumab and ipilimumab combination group exhibited statistically significant higher risks of PMR (RR 2.6, P < 0.0001), GCA (RR 2.9, P 0.0003), and ischemic optic neuropathy (RR 3, p 0.0017). Post-matching, the risk of PMR remained significantly elevated in the nivolumab and ipilimumab combination group (RR 3.8, P < 0.0001), while elevated risks of GCA and ischemic optic neuropathy in the same group lacked statistical significance (Table 2).
Conclusion: Our study underscores the importance of evaluating risks associated with immune checkpoint inhibitors. Increased risks of irAEs in a combination group can be explained by mechanisms of altering both PD-1 and CTLA-4 pathways. Although the overall number of patients with side-effects was low, the findings highlight the need for vigilant monitoring and early detection of irAEs, particularly in patients receiving combination therapy. Given the limitations inherent in retrospective database analyses and the complexity of autoimmune reactions, further research with larger cohorts and prospective designs is essential to validate these findings and refine risk stratification strategies in oncology practice.
To cite this abstract in AMA style:
Tskhakaia I, Lau A. Exploring Risks of Polymyalgia Rheumatica (PMR), Giant Cell Arteritis (GCA) and Complications While Receiving Immune Checkpoint Inhibitors: Comparative Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/exploring-risks-of-polymyalgia-rheumatica-pmr-giant-cell-arteritis-gca-and-complications-while-receiving-immune-checkpoint-inhibitors-comparative-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-risks-of-polymyalgia-rheumatica-pmr-giant-cell-arteritis-gca-and-complications-while-receiving-immune-checkpoint-inhibitors-comparative-analysis/