Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Although hydroxychloroquine (HCQ) is strongly recommended in systemic lupus (SLE) pregnancy, the percentage of pregnant patients with SLE taking HCQ appears highly variable across settings. Little is known about why HCQ is not started or continued for many of these patients. Our study evaluated documented decision-making by patients and providers around this issue.
Methods: We identified 38 deliveries from patients with definite or suspected SLE at a single institution (2008-2016) who reported not using HCQ at their earliest prenatal visit in a retrospective cohort. Prescription orders and physician notes on SLE treatment before/during pregnancy were extracted by chart review, starting 1 year prior to the first documented prenatal visit through to the delivery date. Notes were studied and organized into a framework of explicit and implicit themes.
Results: Overarching concepts were patients’ history of HCQ use, whether patients or providers drove decision-making, and providers’ specialties. In this case series, 84.2% had no exposure to HCQ during the entire pregnancy. Of the 6 patients who started HCQ after their first prenatal visit, the majority were in the second trimester. 39.5% discontinued HCQ within a year prior to their first prenatal visit or early in pregnancy, largely due to conception or maternal/fetal safety concerns. Decision-making around HCQ use was more often physician-led than patient-led (34.2% vs 18.4%), although in 47.4% of cases the issue was not specifically documented within notes about patients’ SLE treatment. Most patients’ records (86.8%) included notes from an obstetrician-gynecologist, 26.3% had notes from a rheumatologist, and 21.1% had notes from a different specialty.
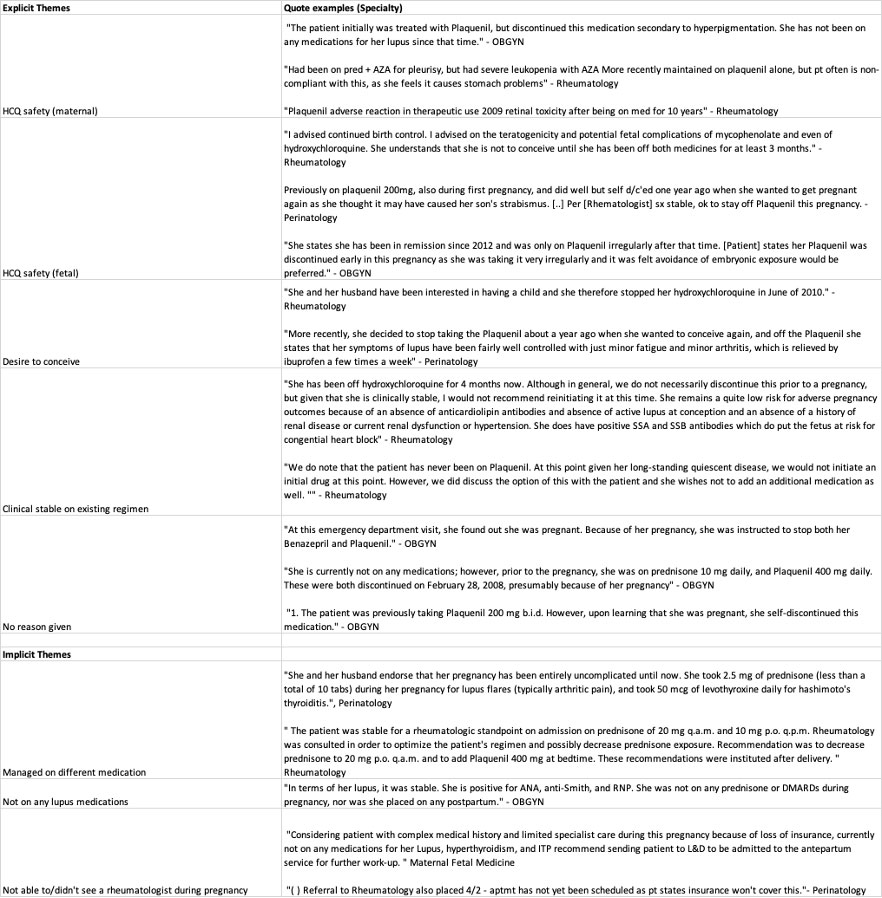
When HCQ use was explicitly discussed in clinical notes, three major themes were identified: (1) ability to conceive on HCQ, (2) concerns about maternal/fetal safety, and (3) reluctance to change a stable patient’s treatment regimen (Table 1). When not explicitly discussed, implicit themes included use of alternate medications, being off SLE treatment entirely, and difficulty seeing a rheumatologist (Table 1). A limitation of our study was lack of external notes, as many patients received SLE/obstetric care not documented firsthand in the medical record.
Conclusion: In this case series of pregnant patients with SLE not on HCQ at their first prenatal visit, over a quarter had previously discontinued HCQ for reasons related to pregnancy, while few ultimately started the medication before delivery. Providers frequently noted that a patient’s clinical stability without HCQ influenced the decision to defer treatment. Understanding these documented clinical decision points may help providers engage in better shared decision-making about HCQ with patients, and may inform researchers using observational data about potential sources of confounding and selection bias.
To cite this abstract in AMA style:
Chan A, Hirz A, Chaichian Y, Rector A, Druzin M, Simard J. Exploring Reasons for Non-Use of Hydroxychloroquine in SLE Pregnancy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/exploring-reasons-for-non-use-of-hydroxychloroquine-in-sle-pregnancy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-reasons-for-non-use-of-hydroxychloroquine-in-sle-pregnancy/