Session Information
Date: Tuesday, November 12, 2019
Title: 5T114: RA – Diagnosis, Manifestations, & Outcomes IV: Outcomes (2846–2851)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
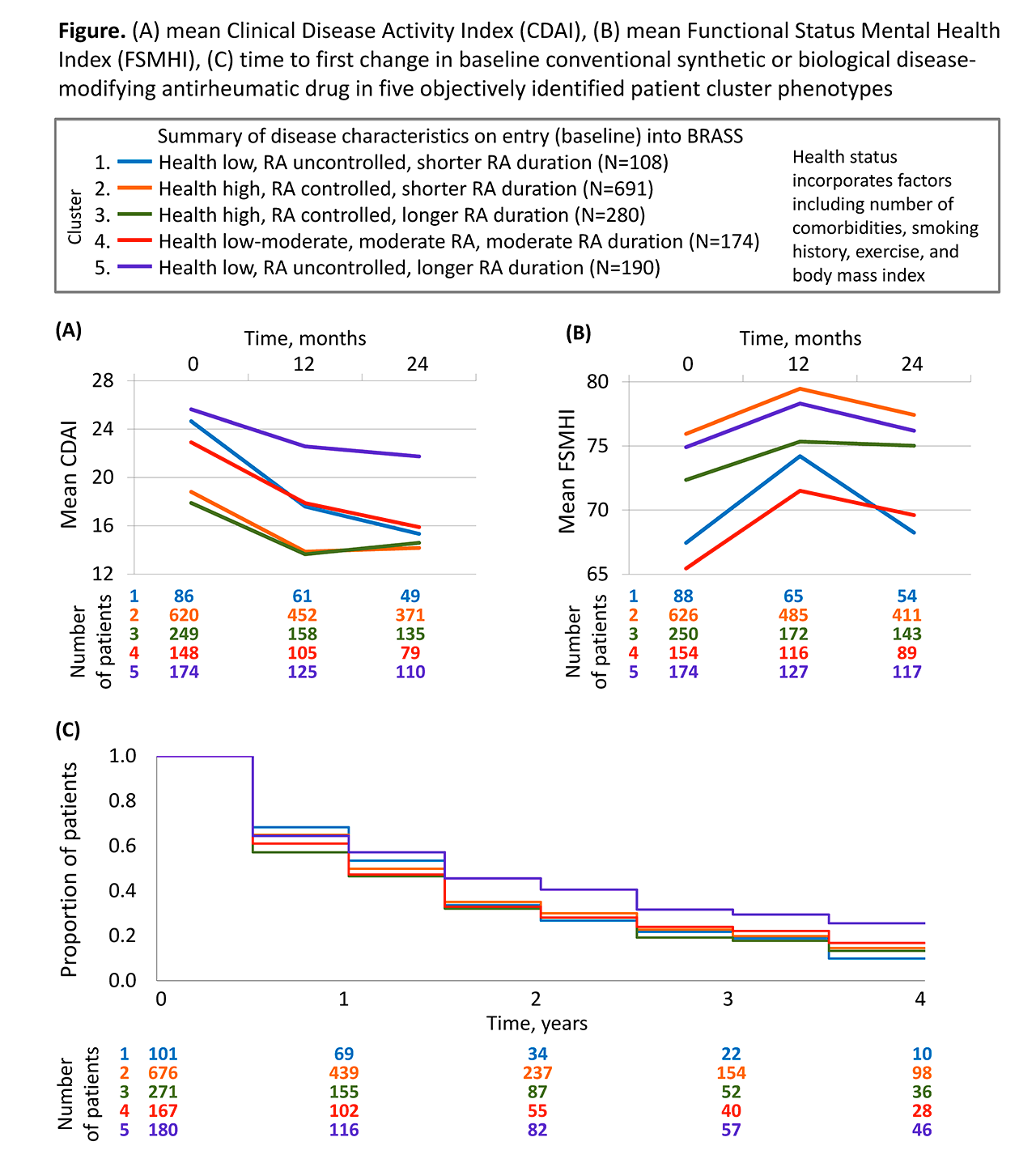
Background/Purpose: Patients with rheumatoid arthritis (RA) may share characteristics that relate to their future outcomes. We investigated clinical outcomes over a 4-year follow-up period in objectively identified RA patient clusters derived empirically via a data-driven approach using the BRASS registry.
Methods: Patient clusters were identified by principal components (PC) and cluster analysis of demographic, socio-economic, health and disease characteristics using patient data collected at entry (baseline) into the BRASS registry. Patients in BRASS are followed in the clinic at least annually and are sent questionnaires at 6-month intervals. Mean score of clinical measures were observed at 12- and 24-months of follow-up including Clinical Disease Activity Index (CDAI), Disease Activity Score 28-joint count C-reactive protein (DAS28-CRP), BRASS self-administered Rheumatoid Arthritis Disease Activity Index (RADAI), swollen and tender joint count (SJC and TJC), Multidimensional Health Assessment Questionnaire (MDHAQ), and Functional Status Mental Health Index (FSMHI). Time to first infection and to first RA medication change over 4 years were analysed via Kaplan-Meier curves.
Results: PC analysis of variables among 1443 patients recorded at entry into BRASS identified 41 PCs that capture the fundamental characteristics involved in RA. These PCs informed the identification of 5 novel patient clusters. Cluster 1 patients (“health low, RA uncontrolled, shorter RA duration”) exhibited the greatest reduction in TJC. Cluster 2 patients (“health high, RA controlled, shorter RA duration”) remained free of infection longer than other clusters. Cluster 3 patients (“health high, RA controlled, longer RA duration”) sustained the lowest mean SJC throughout follow-up. Cluster 4 patients (“health low–moderate, moderate RA, moderate RA duration”) exhibited the greatest improvement in mental health (FSMHI; Figure). Cluster 5 patients (“health low, RA uncontrolled, longer RA duration”) exhibited the highest CDAI scores (Figure) and the highest persistence of therapies at baseline without change.
Conclusion: Five patient clusters identified by data-driven PC analysis of the BRASS registry exhibited distinct patterns of clinical outcomes and management over 4 years. The clinical outcomes data suggest the clusters represent clinically meaningful profiles of RA and illustrate the potential of data-driven patient profiling as a tool to support personalized medicine in RA. Validation in an independent dataset is ongoing.
To cite this abstract in AMA style:
Curtis J, Weinblatt M, Saag K, Bykerk V, Charles-Schoeman C, Fiore S, St John G, Kimura T, Zheng S, Bingham C, Wright G, Bergman M, Nola K, Furst D, Shadick N. Exploring Heterogeneity in Rheumatoid Arthritis: Outcomes up to 4 Years of Follow-Up in Patient Clusters Identified by Data-driven Analysis of the BRASS Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/exploring-heterogeneity-in-rheumatoid-arthritis-outcomes-up-to-4-years-of-follow-up-in-patient-clusters-identified-by-data-driven-analysis-of-the-brass-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-heterogeneity-in-rheumatoid-arthritis-outcomes-up-to-4-years-of-follow-up-in-patient-clusters-identified-by-data-driven-analysis-of-the-brass-registry/