Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Pain is the most common symptom reported by patients (pts) with rheumatic disorders, such as RA.1 Improvement in pain with antirheumatic drugs is generally believed to be mediated by anti-inflammatory effects; however, a possible direct effect on pain of new antirheumatic drugs, such as Janus kinase inhibitors, is being debated.2 Here, we used mediation modeling to explore the mechanisms of action of the effects of tofacitinib on pain in pts with RA.
Methods: This post hoc analysis used data at Month (M)3 from pts with active RA enrolled in the 12-month, Phase 3, randomized, placebo (PBO)-controlled ORAL Standard (NCT00853385) trial.3 Pts received tofacitinib 5 or 10 mg twice daily (BID), adalimumab (ADA) 40 mg once every 2 weeks, or PBO (advancing to tofacitinib 5 or 10mg BID at M3), with stable doses of background methotrexate.3 A mediation, or path, analysis was performed, with pain (Visual Analog Scale; 0–100mm) scores at M3 the dependent variable, treatment (tofacitinib 5 or 10 mg BID or ADA) the independent variable, and inflammation (measured by inflammatory markers CRP, ESR, and swollen joint count in 28 joints [SJC28]) a mediator. An indirect effect was defined as the treatment effect on pain over PBO mediated by CRP, ESR, and SJC28. A direct effect (over PBO) was defined as the treatment effect on pain not attributable to CRP, ESR, and SJC28. M3 data were used without imputations.
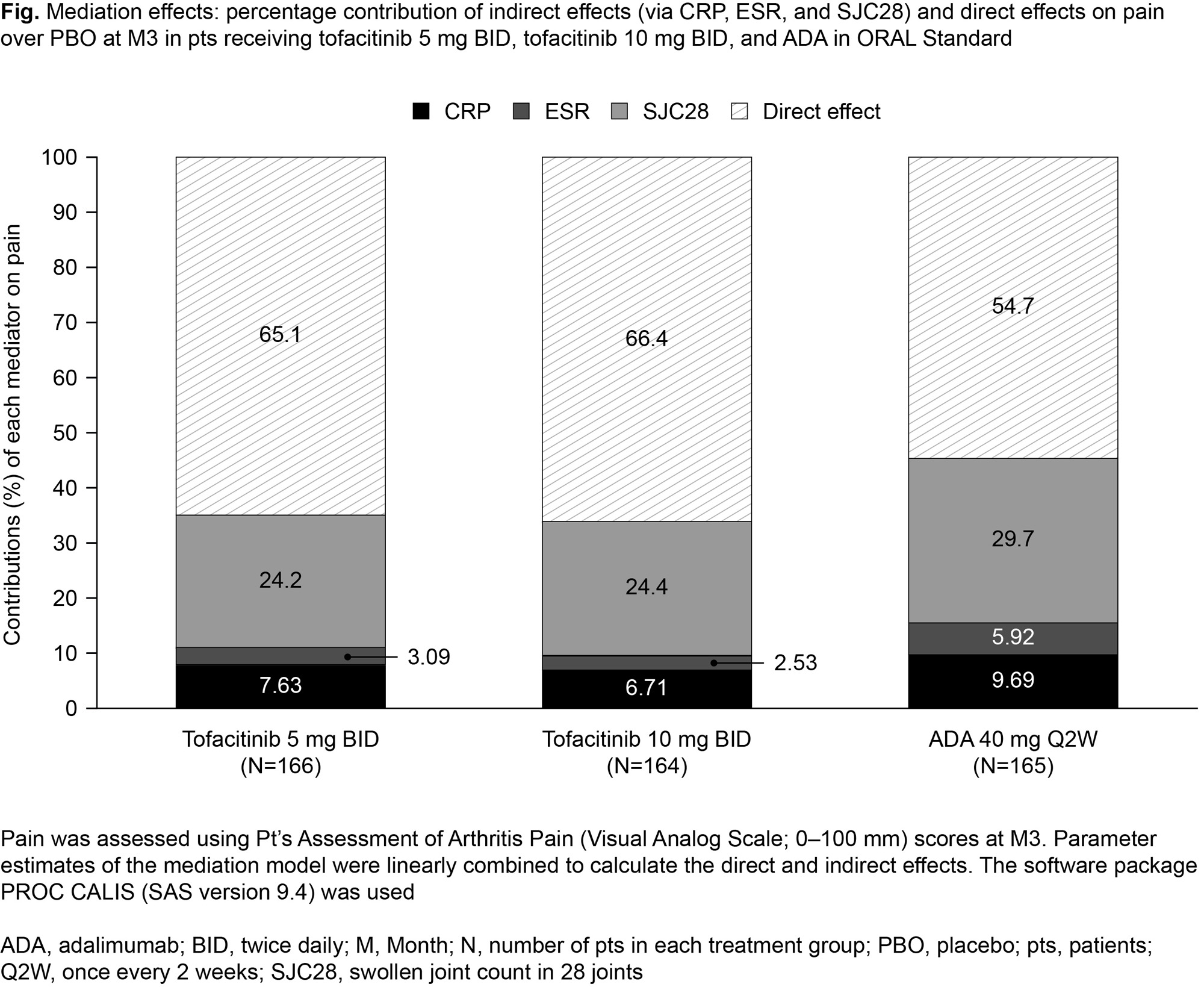
Results: Data for 580 pts were included: 166, 164, 165, and 85 pts received tofacitinib 5mg BID, tofacitinib 10 mg BID, ADA, and PBO, respectively. At M3, the percentage contribution of the indirect effect on pain (ie, attributable to CRP, ESR, or SJC28) over PBO was significant for all treatment arms (all p < 0.001) with the mediation effect being greater for SJC28 vs CRP and ESR (Fig). The percentage contribution of the indirect effect was numerically lower with tofacitinib 5 mg BID (34.9% [95% confidence intervals: 18.1, 51.7]) and 10 mg BID (33.6% [17.1, 50.1]) compared with ADA (45.3% [19.6, 70.9]) (Fig). The percentage contribution of the direct effect on pain over PBO (ie, not attributable to CRP, ESR, or SJC28) was numerically higher with tofacitinib 5 mg BID (65.1% [48.3, 81.9]) and 10 mg BID (66.4% [49.9,82.9]) compared with ADA (54.7% [29.1, 80.4]) (Fig), and significant for all treatment arms (allp < 0.000).
Conclusion: Inflammation, particularly measured by SJC28, was a significant mediator of the effect of tofacitinib 5 and 10 mg BID and ADA on pain. However, the majority of the treatment effects on pain were not attributable to inflammatory changes; this was particularly the case for tofacitinib 5 and 10 mg BID ( > 65%). Therefore, more work is needed to assess whether additional mediators of pain can be found. Limitation: this analysis could only assess variables that were collected in the ORAL Standard study as mediators.
1. American College of Rheumatology Pain Management Task Force. Arthritis Care Res (Hoboken) 2010; 62: 590–9
2. Simon et al. Semin Arthritis Rheum 2021; 51: 278–84
3. Van Vollenhoven et al. N Engl J Med 2012; 367: 508–19
Study sponsored by Pfizer. Medical writing support provided by K Nicholson, CMC Connect; funded by Pfizer.
To cite this abstract in AMA style:
Dougados M, Taylor P, Gruben D, Kessouri M. Exploratory Analysis of the Mechanisms of Action of the Potential Analgesic Effect of Tofacitinib and Adalimumab over Placebo in Patients with Rheumatoid Arthritis: Results of a Mediation Modeling Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/exploratory-analysis-of-the-mechanisms-of-action-of-the-potential-analgesic-effect-of-tofacitinib-and-adalimumab-over-placebo-in-patients-with-rheumatoid-arthritis-results-of-a-mediation-modeling-ana/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploratory-analysis-of-the-mechanisms-of-action-of-the-potential-analgesic-effect-of-tofacitinib-and-adalimumab-over-placebo-in-patients-with-rheumatoid-arthritis-results-of-a-mediation-modeling-ana/