Session Information
Date: Tuesday, November 14, 2023
Title: (1776–1795) Spondyloarthritis Including Psoriatic Arthritis – Basic Science Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Studies in human HLA-B27 transgenic rats largely discredited the idea that CD8+ T cells are key drivers of disease in axial spondyloarthritis (axSpA). However, recent studies in humans have revived interest in the role of CD8+ T cells in axSpA including the description of relevant CD8+ T cell subsets in a number of rheumatic disease settings (CD103+CD49a+ InEx cells, regulatory CD8+KIR+ T cells, Granzyme B and K expressing CD8+ T cell subsets) and the demonstration of expanded TCR clonotypes in the joints and eyes of HLA-B27+ SpA patients. In this study, we used state-of-the-art flow cytometry to search for diagnostic cellular biomarkers in axSpA reporting results for CD8+ T cells.
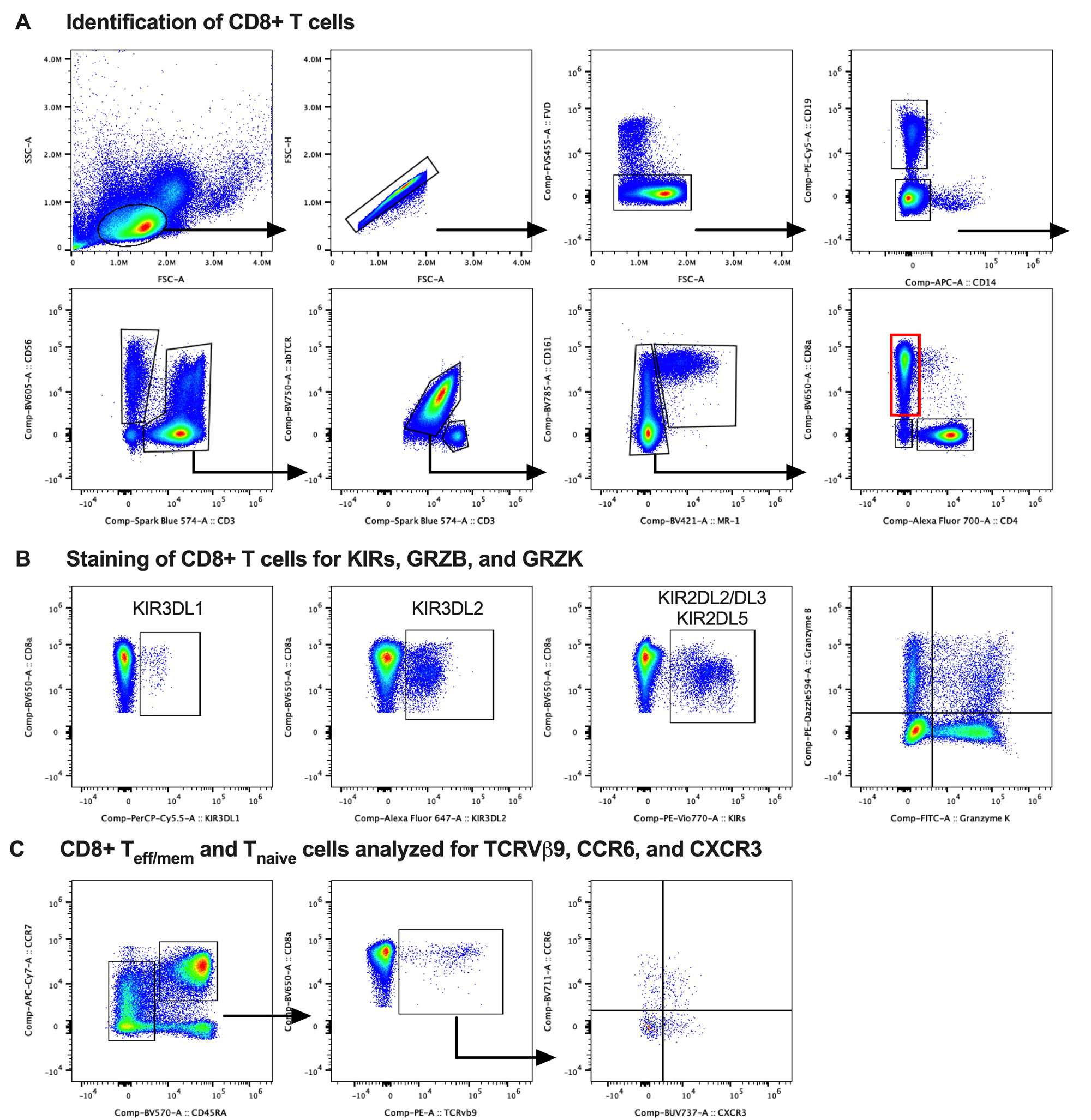
Methods: Study subjects were recruited from our hospital’s Orthopedic and Arthritis Center. Four groups were compared: healthy controls (HC), patients with chronic low back pain without axSpA (cLBP), axSpA patients not on a biologic (axSpA/-), and axSpA patients treated with a TNF inhibitor (axSpA/TNFi). Groups (n=22-23 each) were matched for age, sex and genotyped for HLA-B27. All axSpA patients fulfilled modified New York criteria for ankylosing spondylitis or the 2009 ASAS criteria for axSpA. PBMCs were analyzed in two batches on a CYTEK Aurora spectral flow cytometer. Our customized 30-parameter staining panel included major lineage markers, lymphocyte differentiation and activation markers and functional markers for cytotoxicity, homing, and cytokine production potential. We included antibodies for KIR3DL1 (DX9), KIR3DL2 (DX31), KIR2DL2/L3 (DX27) and KIR2DL5 (UP-R1) as well as TCRVβ9+ (MKB1). Cells were further stained intracellularly for GRZB (GB11) and GRZK (GM26E7). FlowJo was used for quality control and analysis (see gating strategy in Fig. 1).
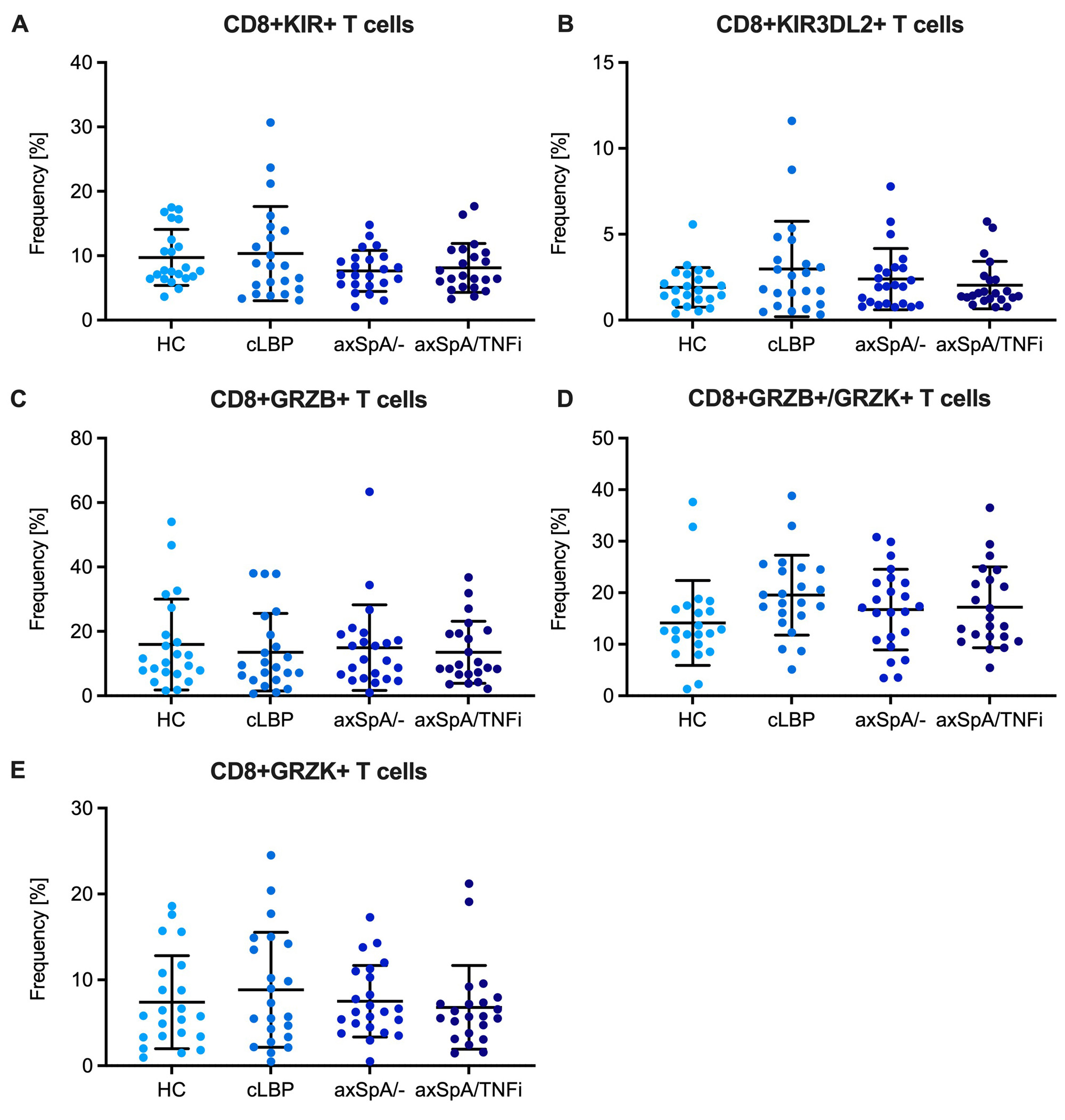
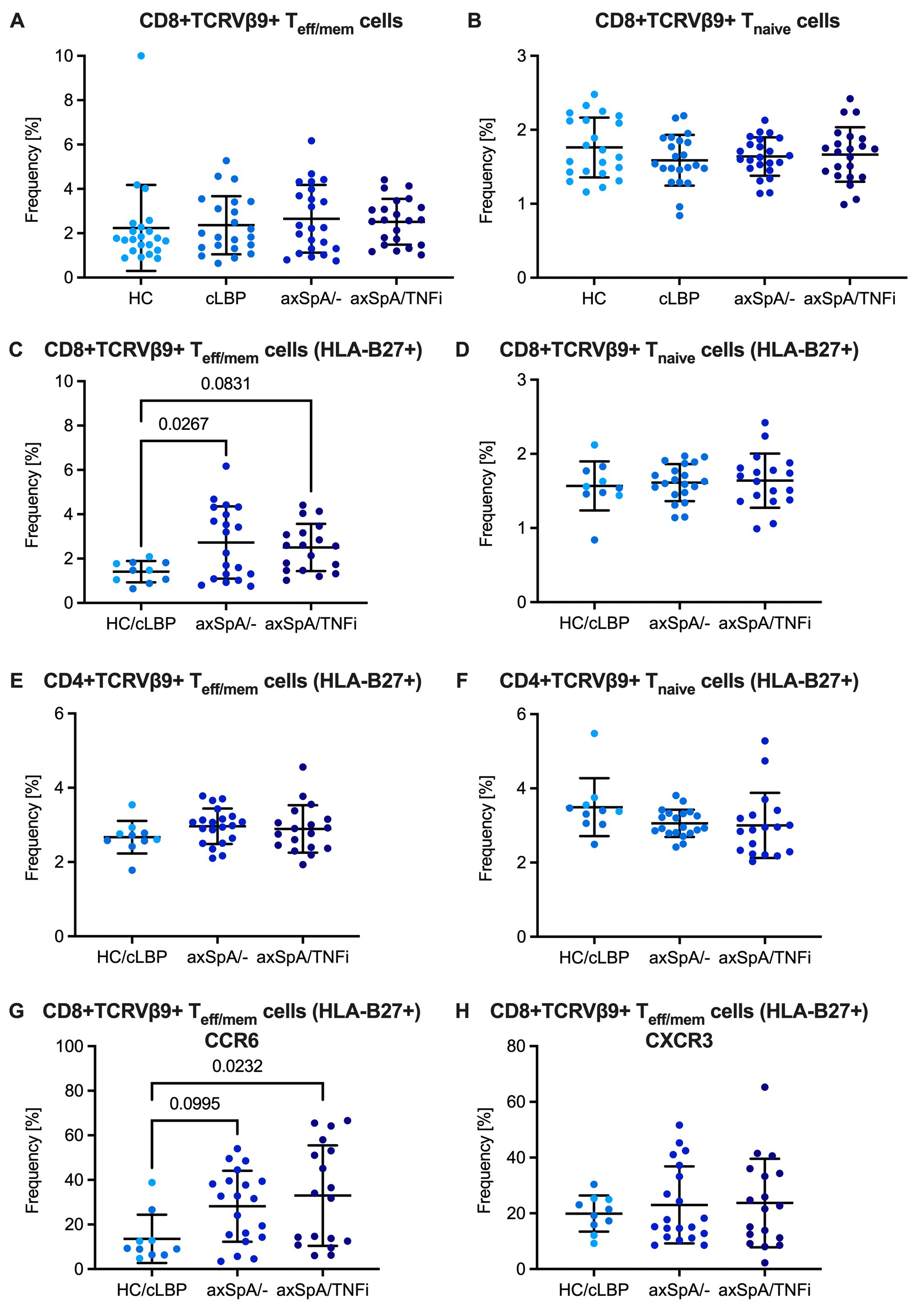
Results: There were no differences in the frequency of CD8+ T cells expressing any KIR or CD8+ T cells expressing the HLA-B27 binding KIR3DL2 between HC, cLBP, axSpA/- and axSpA/TNFi subjects (Fig. 2A,B). There were also no differences in the frequency of CD8+ T cell subsets distinguished by expression of GRZB and GRZK (Fig. 2C-E). About 2% of CD8+CD45RAloTeff/mem and CD8+CD45RAhiCCR7+ Tnaive cells were TCRVβ9 positive without differences between groups. However, when only HLA-B27+ individuals were analyzed, we noticed an expansion of TCRVβ9+ cells amongst CD8+ Teff/mem cells in axSpA/- and axSpA/TNFi subjects, which was not seen in CD8+ Tnaïve cells or in the respective CD4+ T cell subsets (Fig. 3). Compared with control subjects, CD8+TCRVβ9+ Teff/mem cells in axSpA patients showed increased expression of CCR6 suggesting a Tc17 phenotype.
Conclusion: The observed expansion of CD8+Vβ9+ Teff/mem cells in the peripheral blood of HLA-B27+ patients with axSpA is consistent with the previously reported expansion of TRBV9–CDR3–TRBJ2.3 clonotypes in tissues and supports the peptide hypothesis for the HLA-B27 association with axSpA. The in-depth characterization of the CD8+TCRVβ9+ Teff/mem population using additional markers included in the staining panel is ongoing.
To cite this abstract in AMA style:
Bauchiero C, Lefton M, Sinnappan S, Sparks J, Ermann J. Expansion of CD8+ TCRVβ9+ T Cells in the Peripheral Blood of HLA-B27+ Patients with Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/expansion-of-cd8-tcrv%ce%b29-t-cells-in-the-peripheral-blood-of-hla-b27-patients-with-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/expansion-of-cd8-tcrv%ce%b29-t-cells-in-the-peripheral-blood-of-hla-b27-patients-with-axial-spondyloarthritis/