Session Information
Date: Saturday, November 12, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Incomplete lupus erythematosus (ILE) involves clinical and/or serologic manifestations consistent with but insufficient for SLE classification. While a subset of ILE patients transition to SLE, most maintain a relatively mild, stable disease course. However, the nature of ILE is poorly understood, causing difficulties in diagnosis, risk stratification, and treatment. We previously found that ILE patients have fewer traditional autoantibody specificities compared to SLE patients, suggesting that autoantibody accrual may influence the transition to SLE in ILE patients. This study aimed to further characterize the autoantibody profiles of ILE patients in comparison to SLE patients in a racially diverse cohort using ImmunArray iCHIP™.
Methods: ILE (≤3 ACR criteria; n=273) and SLE (≥4 ACR criteria; n=43) patients and unaffected controls (n=40) were selected from existing collections in the Arthritis & Clinical Immunology Biorepository at Oklahoma Medical Research Foundation. Patients were further categorized based on SLICC classification criteria as ILE/ILE (ILE patients with ≤3 SLICC criteria; n = 178), ILE/SLE patients (ILE patients with ≥4 SLICC criteria; n=95), and SLE/SLE (SLE patients with ≥4 SLICC criteria; n=23). ImmunArray iCHIP™ was used to measure 922 (461 IgM and 461 IgG) serum autoantibodies against SLE-associated antigens. A cut-off of 4 standard deviations above the control mean determined autoantibody positivity.
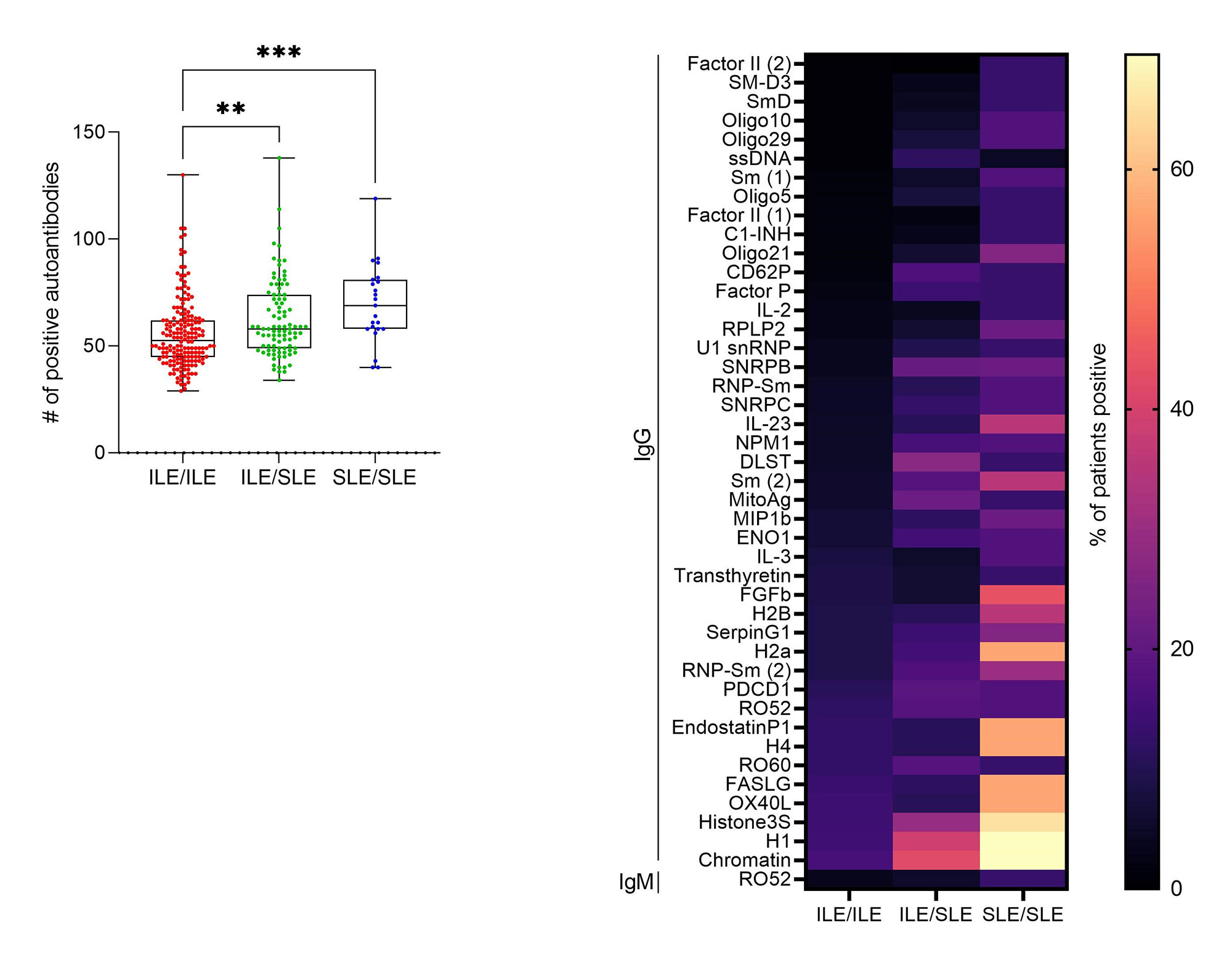
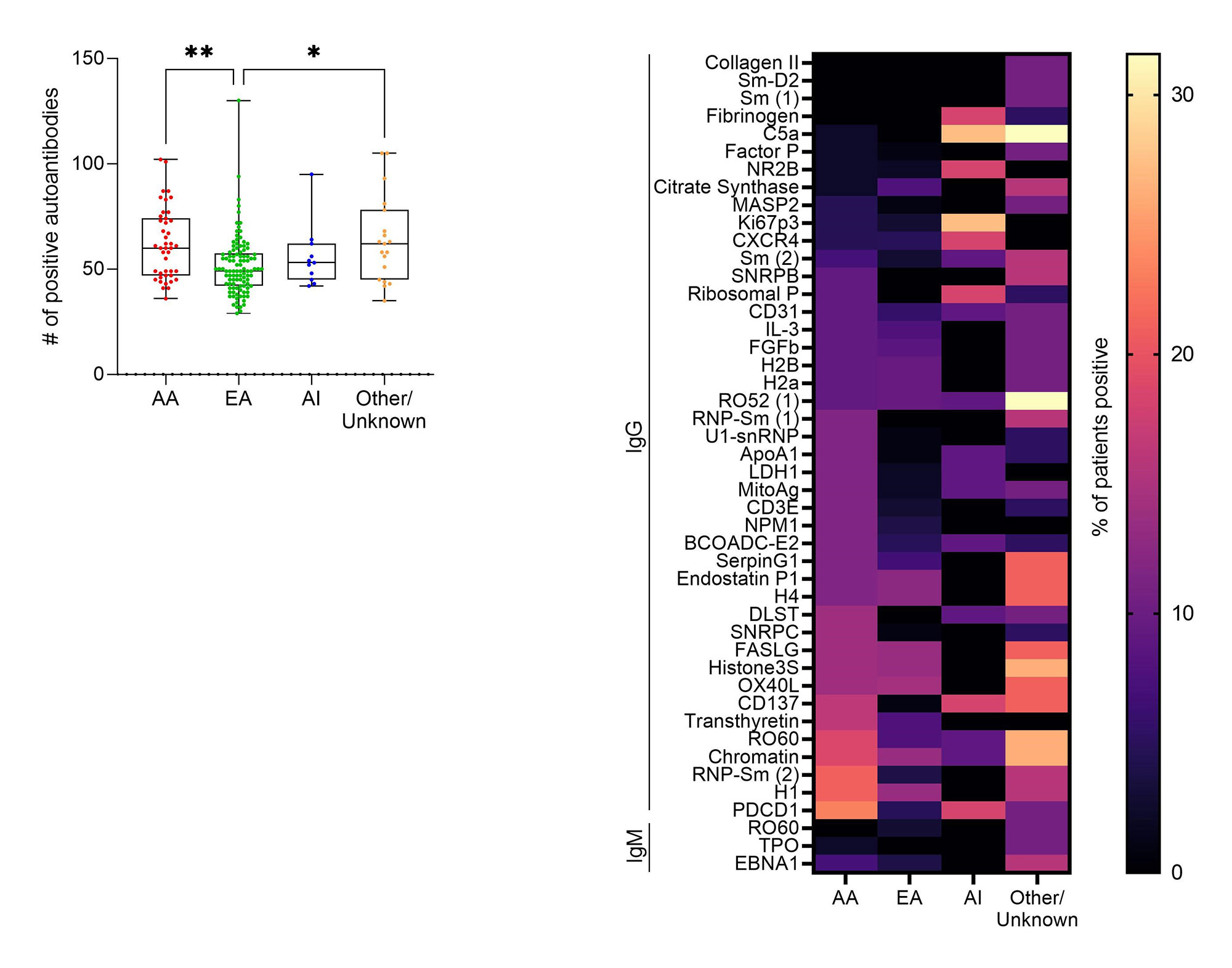
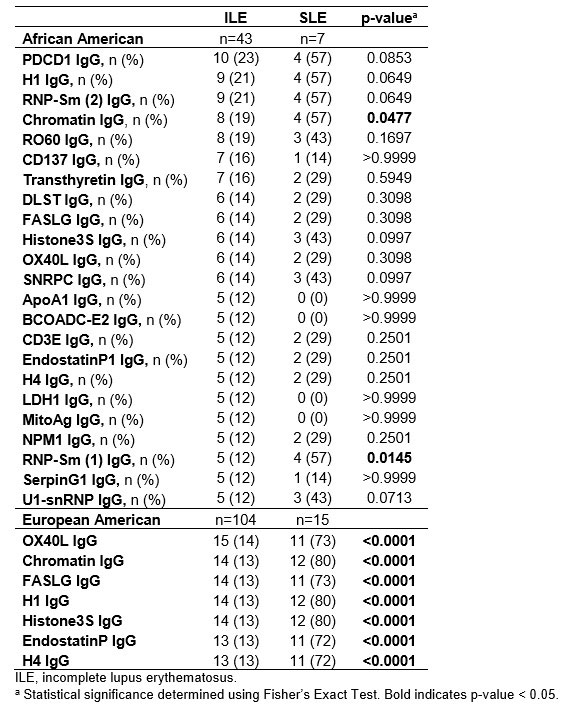
Results: All ILE/ILE, ILE/SLE, and SLE/SLE were positive for at least one autoantibody by the ImmunArray iCHIP. However, ILE/ILE patients had fewer autoantibody specificities compared to ILE/SLE and SLE/SLE patients (Figure 1A). Furthermore, the prevalence of autoantibody positivity was reduced in ILE patients (Figure 1B). Consistent with studies in SLE, African American (AA) ILE patients had significantly more autoantibody specificities compared to European American (EA) ILE patients, and autoantibody profiles varied between races (Figure 2). We next determined which autoantibodies were specific to ILE/ILE by race (Table). Although the autoantibodies targeting ApoA1-IgG, BCOADC-E2-IgG, LDH1-IgG, and MitoAg-IgG were identified in 12% of AA ILE patients and not AA SLE patients, this difference did not reach statistical significance. No unique autoantibody specificities were identified in EA ILE patients compared to EA SLE patients.
Conclusion: ILE/ILE patients have significantly fewer autoantibody specificities compared to ILE/SLE and SLE/SLE patients, and autoantibody prevalence and profiles differ by race. In addition, we identified 4 autoantibodies specific to AA ILE patients and not AA SLE patients, which may help distinguish between SLE and ILE patients and suggests potential therapeutic targets for ILE. However, larger studies are necessary to validate and demonstrate the diagnostic potential of these findings.
To cite this abstract in AMA style:
Wood R, Wagner C, Guthridge C, Guthridge J, Wallace S, Safer P, James J. Expanded Autoantibody Profiling in Incomplete Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/expanded-autoantibody-profiling-in-incomplete-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/expanded-autoantibody-profiling-in-incomplete-lupus-erythematosus/