Session Information
Session Type: Abstract Session
Session Time: 3:00PM-3:15PM
Background/Purpose: Psoriatic arthritis (PsA) is often diagnosed late, leading to worse outcomes. Enthesitis is a key feature that can aid in early detection, but its clinical assessment is unreliable. Ultrasound (US) offers a more objective method for evaluating enthesitis, yet standardization remains a challenge. The GRAPPA Ultrasound Working Group is developing the Diagnostic Ultrasound Enthesitis Tool (DUET), a PsA-specific sonographic enthesitis scoring system to distinguish PsA from non-inflammatory conditions. While DUET has been derived from a discovery cohort, external validation is needed before its clinical adoption. In this study, we aimed to evaluate the discriminative ability of candidate DUET scores using independent data to inform the selection of a DUET scoring system.
Methods: This retrospective cross-sectional study analyzed US scans from PsA patients and non-psoriatic controls collected in a single centre following standard protocol. All PsA patient met the DUET enrollment criteria including PsA duration < 5 years and were not using biologics. Controls had no psoriasis or other autoimmune disease. Enthesis US scans of the quadriceps, proximal and distal patellar tendons, triceps and Achilles were scored by a single reader blinded to the diagnosis for entheseal elementary lesions including hypoechogenicity, thickening, Doppler signal, enthesophytes, calcifications and erosions. We evaluated seven candidate DUET scores, each incorporating different combinations of entheseal sites and elementary lesions. Models were refitted using coefficients estimated in the discovery cohort. Model performance was also stratified by age group.
Results: In our validation cohort of 102 PsA patients and 69 controls, the seven candidate DUET risk scores yielded areas under the curve (AUCs) ranging from 0.660 to 0.764 (Figure 1). Stratifying the risk scores by age with a cutoff of 55 years enhanced AUCs to 0.781 (under 55) and 0.753 (over 55) (Figure 2).
Conclusion: The candidate DUET scores demonstrated discriminative ability in an external validation cohort, with additional improvement following age stratification. These findings will guide further refinement of the selected DUET score and inform its integration into clinical and research settings.
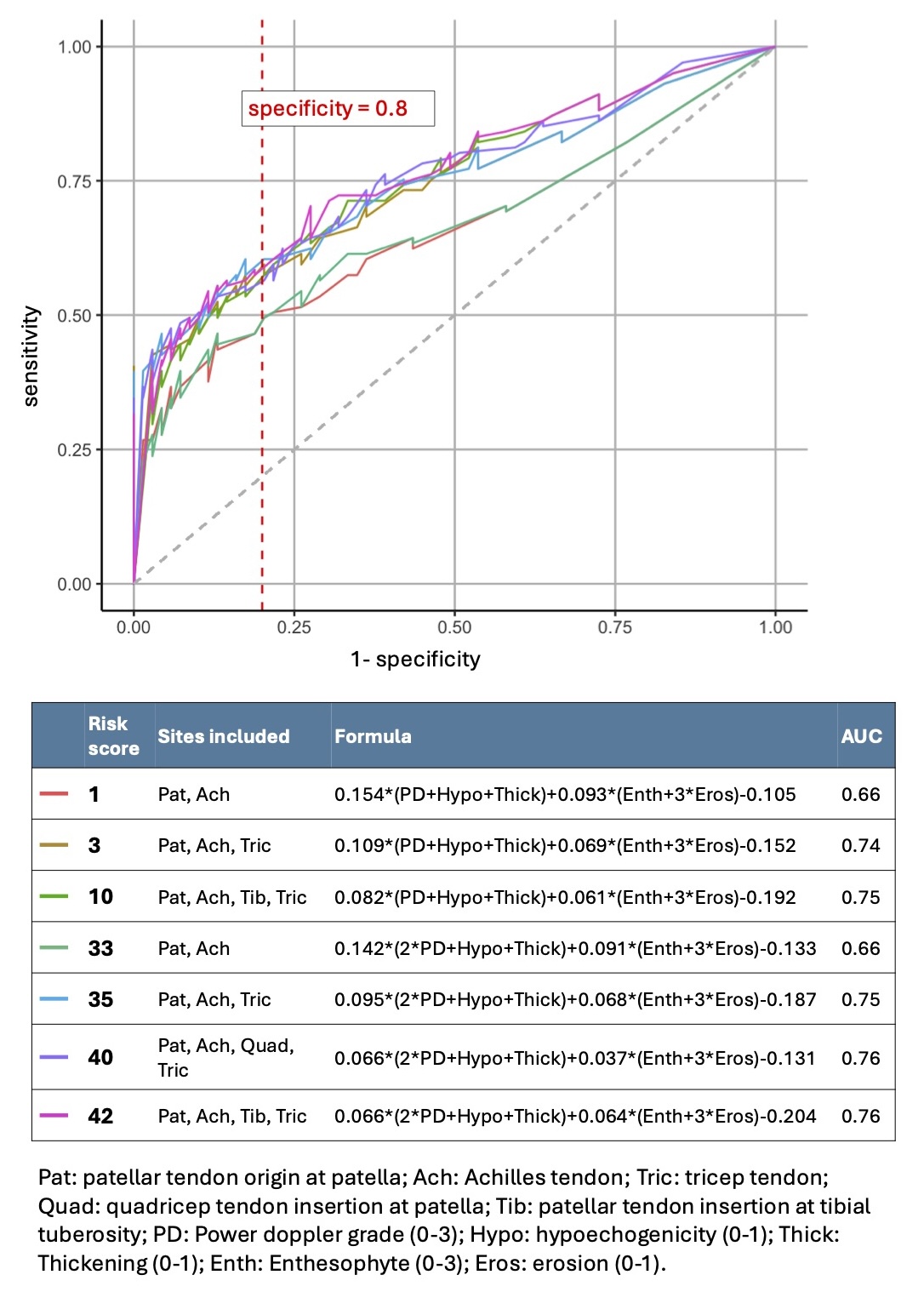 Figure 1. ROC curve of candidate DUET scores
Figure 1. ROC curve of candidate DUET scores
.jpg) Figure 2. Discriminative ability of candidate DUET scores stratified by age groups
Figure 2. Discriminative ability of candidate DUET scores stratified by age groups
To cite this abstract in AMA style:
Nguyen N, Yang M, Aydin S, Kaeley G, Cook R, Eder L. Evaluation of the Performance of Candidate Enthesitis Ultrasound Scoring Systems for Psoriatic Arthritis Diagnosis – DUET Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-performance-of-candidate-enthesitis-ultrasound-scoring-systems-for-psoriatic-arthritis-diagnosis-duet-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-performance-of-candidate-enthesitis-ultrasound-scoring-systems-for-psoriatic-arthritis-diagnosis-duet-study/
