Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Ianalumab, a glycoengineered, fully human IgG1 mAb directed against B cell-activating factor (BAFF)-receptor (BAFF-R), targets B cells and their functions through dual mechanism of action: depletion of B cells through enhanced antibody-dependent cellular cytotoxicity and blockade of BAFF:BAFF-R–mediated signals. Here we present a multi-modal ultrasound (US) assessment of major salivary glands (SG) to better understand the mode of action of ianalumab on parotid (PGs) and submandibular glands (SMGs) in patients with Sjögren’s disease (SjD).
Methods: This is an open-label, single-center, non-randomized, phase 2 mechanistic study (NCT05124925) with a 5-week screening period, a 6-month treatment period, and a follow up period of up to 2 years after the last dose. Eligible participants included those who fulfilled the 2016 ACR/EULAR classification criteria for SjD with anti-Ro/SSA autoantibodies, EULAR Sjögren’s Syndrome Patient Reported Index score of ≥5, labial SG focus score (FS) of ≥0.3/4mm2, and B/B+T cell ratio of ≥0.2 at screening. This interim analysis reports the results of patients who completed the 24-week treatment period (ianalumab 300 mg monthly subcutaneous injection). As secondary outcome of the study, multimodal US assessment of major SGs was evaluated at W25 compared with BL. PGs and SMGs were assessed individually and analyzed by pairs for the following SGUS parameters: 1) B-mode (size and OMERACT score1), 2) Power Doppler (macrovascularization scaling and index), 3) contrast-enhanced US (CEUS) (microvascularization parameters derived from microbubbles injection for PGs only) and 4) sonoelastography (stiffness).
Results: In the safety analysis set (Nf21), ianalumab was well-tolerated with no drug-related serious adverse events (AEs) and one AE leading to discontinuation. SGUS assessment and contrast agent administration were well tolerated. Overall, 17 participants completed the 24-week open-label treatment, underwent paired screening and W25 labial SG biopsy, and were analyzed. Figure 1 displays multimodal SGUS acquisitions from a single patient pre and post treatment. Overall, BL SGUS features indicated moderate glandular involvement, with mean OMERACT scores of 2.53 and 2.24, and Doppler Vascularization Scaling of 1.03 and 0.74, in PGs and SMGs, respectively (Table 1). At W25, B-mode, Doppler and CEUS vascularization and elastography stiffness parameters numerically decreased, and to a larger extent in the PGs compared with SMGs (Table 1). Notably, the mean OMERACT score reduced by -0.41 and -0.24, and the mean Doppler Scaling reduced by -0.26 and -0.24, in PGs and SMGs, respectively. The reduction in tissue perfusion was further supported by CEUS measurements derived from the time intensity curve observed in response to the microbubble injection, as shown for the left PGs in Figure 2.
Conclusion: Patients treated with ianalumab 300 mg monthly showed improvement in PGs structure and vascularization at W25 vs BL using multimodal ultrasound assessment. Innovative measures with CEUS display a good sensitivity to change after treatment and should be considered in future clinical trials. Reference 1. Jousse-Joulin et al. Ann Rheum Dis. 2019;78:967-973
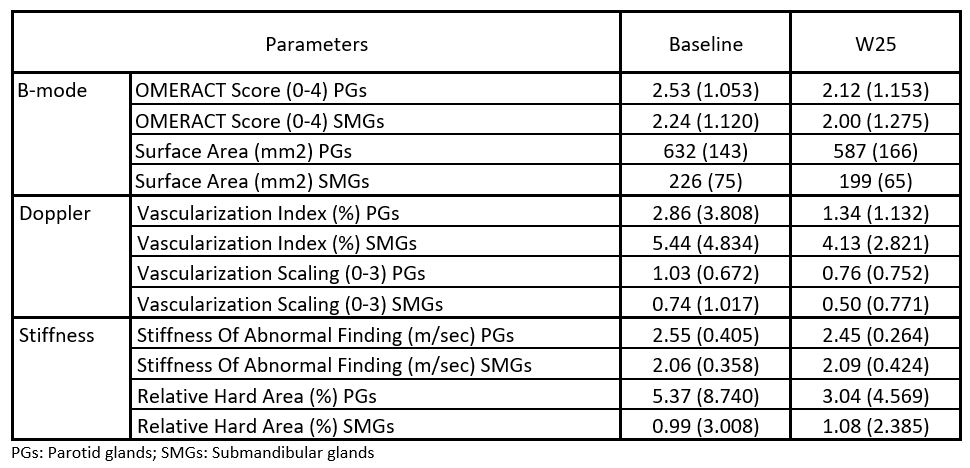 Figure 1. Multimodal SGUS from a single patient at BL and W25
Figure 1. Multimodal SGUS from a single patient at BL and W25
.jpg) Table 1. Mean (SD) SGUS parameters at BL and W25
Table 1. Mean (SD) SGUS parameters at BL and W25
.jpg) Figure 2. Left parotid CEUS parameters at BL and W25
Figure 2. Left parotid CEUS parameters at BL and W25
To cite this abstract in AMA style:
jousse s, Devauchelle V, quere b, Cornec D, Denis C, MARHADOUR T, Tison A, Baley P, SARAUX A, Giglioli N, SIPS C, Chen S, Hillenbrand R, Siegel R, Hueber W, Bonal C, Laurent D. Evaluation of the Ianalumab Treatment Effects on Major Salivary Glands of Patients With Sjögren’s Disease by Multimodal Ultrasound: Results From a Phase 2 Mechanistic Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-ianalumab-treatment-effects-on-major-salivary-glands-of-patients-with-sjogrens-disease-by-multimodal-ultrasound-results-from-a-phase-2-mechanistic-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-ianalumab-treatment-effects-on-major-salivary-glands-of-patients-with-sjogrens-disease-by-multimodal-ultrasound-results-from-a-phase-2-mechanistic-study/
